Suppose that 50.0 mL of 0.25 m CH 3 NH 2 (aq) is titrated with 0.35 m
Question:
Suppose that 50.0 mL of 0.25 m CH3NH2(aq) is titrated with 0.35 m HCl(aq).
(a) What is the initial pH of the 0.25 m CH3NH2(aq)?
(b) What is the pH after the addition of 15.0 mL of 0.35 m HCl(aq)?
(c) What volume of 0.35 m HCl(aq) is required to reach half way to the stoichiometric point?
(d) Calculate the pH at the halfway point.
(e) What volume of 0.35 m HCl(aq) is required to reach the stoichiometric point?
(f) Calculate the pH at the stoichiometric point.
(g) Use Table 6H.2 to select an indicator for the titration.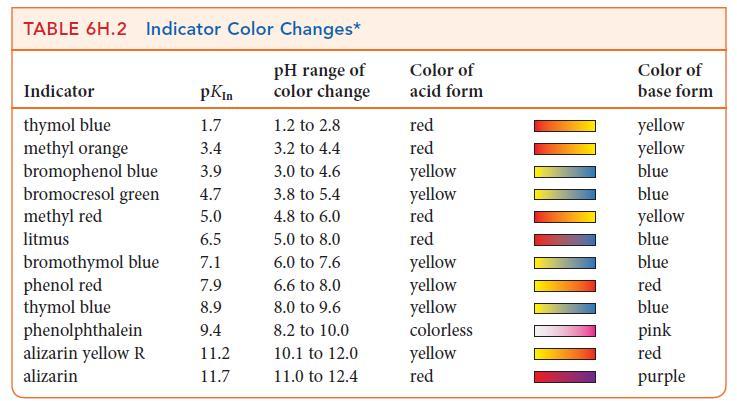
Transcribed Image Text:
TABLE 6H.2 Indicator Color Changes* pH range of color change Indicator thymol blue methyl orange pKin 1.7 3.4 bromophenol blue 3.9 4.7 bromocresol green methyl red 5.0 litmus 6.5 7.1 7.9 8.9 9.4 11.2 11.7 bromothymol blue phenol red thymol blue phenolphthalein alizarin yellow R alizarin 1.2 to 2.8 3.2 to 4.4 3.0 to 4.6 3.8 to 5.4 4.8 to 6.0 5.0 to 8.0 6.0 to 7.6 6.6 to 8.0 8.0 to 9.6 8.2 to 10.0 10.1 to 12.0 11.0 to 12.4 Color of acid form red red yellow yellow red red yellow yellow yellow colorless yellow red Color of base form yellow yellow blue blue yellow blue blue red blue pink red purple
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a Initial pH of 025 M CH3NH2 CH3NH2aq H2O1CH3NH3 aq OHaq K 36 10 CH3NH3 OH CH3NH2 Concentration mol ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Suppose that 15.0 mL of 0.15 m NH 3 (aq) is titrated with 0.10 m HCl(aq). (a) What is the initial pH of the 0.15 m NH 3 (aq)? (b) What is the pH after the addition of 15.0 mL of 0.10 m HCl(aq)? (c)...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A set of 2M biorthogonal signals is obtained from a set of M orthogonal signals by augmenting it with the negative of each signal in the set. (a) The extension of orthogonal to biorthogonal signals...
-
A wave with frequency of 1200 Hz propagates along a wire that is under a tension of 800 N. The wavelength of the wave is 24 cm. What will be the wavelength if the tension is decreased to 600 N and...
-
a. It is now January 1. You plan to make 5 deposits of $100 each, one every 6 months, with the first payment being made today. If the bank pays a nominal interest rate of 12 percent but uses...
-
7. Suppose that f : R2 -+ R2 has continuous first-order partial derivatives in some ball Br(xo, Yo), r > o. Prove that if 6. j (xo, Yo) i= 0, then 8f11 (f( )) = 8h/8y(xo, Yo) uJ;lX xo, Yo tA.. ..J(....
-
What are typical maturities, denominations, and interest payments of a corporate bond? What mechanisms protect bondholders?
-
need pls Tido co produces dogfood. All materials are considered direct costs everything is considered overheads The accounting department has prepared the following worksheets to prepara management...
-
Based on a 2018 study, the average elapsed time between when a user navigates to a website on a mobile device until its main content is available was 14.6 seconds. This is more than a 20% increase...
-
Which is the stronger acid, hydrocyanic acid, HCN, or the ammonium ion, NH 4 ? Justify your answer.
-
Zinc(II) readily forms the complex ion Zn(OH) 4 2 . Explain how this fact can be used to distinguish a solution of ZnCl 2 from MgCl 2 .
-
Xr10-28 One of the few negative side effects of quitting smoking is weight gain. Suppose that the weight gain in the 12 months following a cessation in smoking is normally distributed with a standard...
-
Haley Romeros had just been appointed vice president of the Rocky Mountain Region of the Bank Services Corporation (BSC). The company provides check processing services for small banks. The banks...
-
Draw a simple but complete hydraulic circuit diagram to drive two actuators, one of which must be connected to a pressure reducing valve to control its pressure because of the delicacy of the task...
-
1. What specific skills would a person have to be a successful director for a parks and recreation position? 2. What experience would a person have working with an elected board for parks and...
-
Required labor time per unit ( hours ) Maximum demand ( units ) Contribution margin per unit Product M 2 6 , 5 0 0 $ 5 . 0 0 Product N 3 8 , 0 0 0 $ 5 . 7 0 If Bush uses the most effective approach...
-
ansewr pls Repeat Exercise 5.8.1 using (a) one rectangle; (b) four rectangles. Data From Exercise 5.8.1 A square plate size \(100 \mathrm{~cm} \times 100 \mathrm{~cm}\) is subjected to an isothermal...
-
A two-way set-associative cache has lines of 16 bytes and a total size of 8 kbytes. The 64-Mbyte main memory is byte addressable. Show the format of main memory addresses.
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
Amphotericin B is a powerful antifungal agent used for intravenous treatment of severe fungal infections. Identify the most acidic proton in this compound: , Amphotericin B NH2
-
Predict the position of equilibrium for each of the following reactions: (a) (b) (c) HO NH NH2
-
As we will learn in Chapter 21, treating a lactone (a cyclic ester) with sodium hydroxide will initially produce an anion: This anion rapidly undergoes an intramolecular proton transfer, in which the...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


