The heat capacity of liquid iodine is 80.7 J K21 mol 1 , and its
Question:
The heat capacity of liquid iodine is 80.7 J · K21 · mol–1, and its enthalpy of vaporization is 41.96 kJ · mol–1 at its boiling point (184.3 °C). Using these facts and information in Appendix 2A, calculate the enthalpy of fusion of iodine at 25 °C.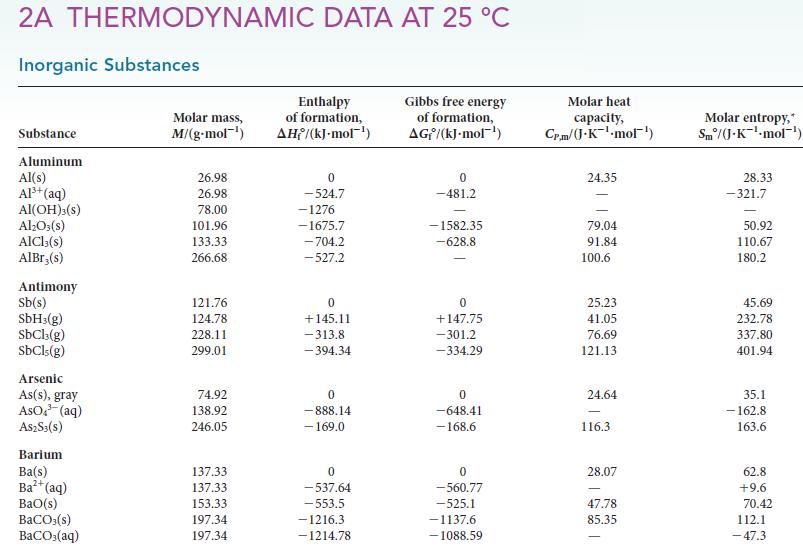
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) AI(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
12...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Suppose you were investigating the correlation between intermolecular forces and the way in which molecules cohere to each other in a liquid and decided that you can gain some insight by comparing...
-
Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 C to hydrazine vapor at its normal boiling point. Integrative Example Use...
-
The heat of vaporization of water at the normal boiling point, 373.2 K, is 40.66 kJ/mol. The specific heat capacity of liquid water is 4.184 JK-1g-1 and of gaseous water is 2.02 J K-1g-1. Assume that...
-
One analogy that is used to think about what is happening in an electric circuit is that of a bucket brigade. Bucket brigades were used before 1900 to fight fires. A group of people would each have a...
-
Daly Inc. reported the following data: Net income $225,000 Depreciation expense 25,000 Gain on disposal of equipment 20,500 Decrease in accounts receivable 14,000 Decrease in accounts payable 3,600...
-
Given the unity feedback system of Figure P6.3 with tell how many closed-loop poles are located in the right half-plane, in the left half-plane, and on the j-axis. 8 G(s) s(s6 255 - s4 + 2s3 + 4s2 -...
-
5 What is the main result of each of the three phases of the strategic marketing process? (a) planning, (b) implementation, and (c) evaluation.
-
A particle moves in a spherically symmetric force field with potential energy given by U(r) = k/r. Calculate the Hamiltonian function in spherical coordinates, and obtain the canonical equations of...
-
Accounting Rate of Return Each of the following scenarios is independent. Assume that all cash flows are after-tax cash flows. a. Cobre Company is considering the purchase of new equipment that will...
-
(a) Calculate the change in entropy of a block of copper at 25C that absorbs 65 J of energy from a heater. (b) If the block of copper is at 100. C and it absorbs 65 J of energy from the heater, what...
-
Consider the enthalpies of fusion and the melting points of the following elements: Pb, 5.10 kJ mol 1 , 327 C; Hg, 2.29 kJ mol 1 , 239 C; Na, 2.64 kJ mol 1 , 98 C. Given these data, determine...
-
Supposing your company intends to set up a subsidiary in Thailand. Which countrys nationals do you pick up to head the subsidiary India, Thailand or any other country?
-
For the demand equation, express the total revenue R as a function of the price p per item. R(p) q=-6p+ 600 Sketch the graph of the resulting function. 20000 19000 18000 17000 16000 O 15000 14000...
-
Kosovski Company is considering Projects S and L, whose cash flows are shown below. These projects are mutually exclusive, equally risky, and repeatable. The WACC is 11.50%. Year: 0 1 2 3 4 CF for S:...
-
What is among the most important things you should do in a negotiation? What is among the most important things you should do in a negotiation? Try to get your way on as many issues as possible. Find...
-
analyze the following column values and answer question: Value Value Label Frequency Percentage Weighted Percentage 1 - 87 Number of children Notes: _ _ = Number of children 113,819 25.78 36.41 88...
-
Reflect on the following questions. Post your response to the discussion board. Post your discussion post by Thursday . 1 peer response due by Sunday. This discussion has two parts: 1) Mediators and...
-
From the standard reduction potentials listed in Table 19.1 for Zn/Zn2+ and Cu+/Cu2+, calculate ÎG° and the equilibrium constant for the reaction Zn(s) + 2Cu-"(aq)- Zn2+ (aq) + 2Cu+(aq)...
-
Medi-Exam Health Services, Inc. (MEHS), located in a major metropolitan area, provides annual physical screening examinations, including a routine physical, EKG, and blood and urine tests. MEUS's...
-
Calculate the impact rate on a 1.00-cm 2 section of a vessel containing oxygen gas at a pressure of 1.00 atm and 27C.
-
A sample of solid potassium chlorate (KClO 3 ) was heated in a test tube (see Fig. 5.10) and decomposed according to the following reaction: The oxygen produced was collected by displacement of water...
-
The mole fraction of nitrogen in air is 0.7808. Calculate the partial pressure of N 2 in air when the atmospheric pressure is 760. torr.
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


