The rate constant of the reaction O(g) + N 2 (g) NO(g) + N(g), which takes
Question:
The rate constant of the reaction O(g) + N2(g) → NO(g) + N(g), which takes place in the stratosphere, is 9.7 * 1010 L · mol–1 · s–1 at 800. °C. The activation energy of the reaction is 315 kJ · mol–1. What is the rate constant at 700. °C? (See Box 7E.1.)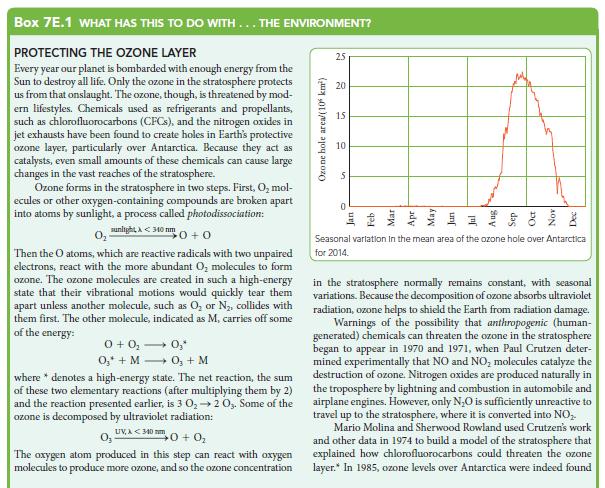
Transcribed Image Text:
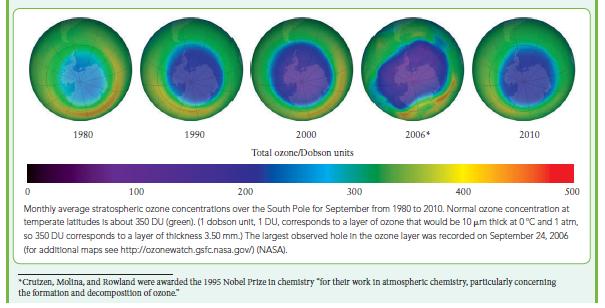
Box 7E.1 WHAT HAS THIS TO DO WITH... THE ENVIRONMENT? PROTECTING THE OZONE LAYER Every year our planet is bombarded with enough energy from the Sun to destroy all life. Only the ozone in the stratosphere protects us from that onslaught. The ozone, though, is threatened by mod- ern lifestyles. Chemicals used as refrigerants and propellants, such as chlorofluorocarbons (CFCs), and the nitrogen oxides in jet exhausts have been found to create holes in Earth's protective ozone layer, particularly over Antarctica. Because they act as catalysts, even small amounts of these chemicals can cause large changes in the vast reaches of the stratosphere. Ozone forms in the stratosphere in two steps. First, O₂ mol- ecules or other oxygen-containing compounds are broken apart into atoms by sunlight, a process called photodissociation: sunlight A<340 nm 0 +0 Then the O atoms, which are reactive radicals with two unpaired electrons, react with the more abundant O₂ molecules to form ozone. The ozone molecules are created in such a high-energy state that their vibrational motions would quickly tear them apart unless another molecule, such as O₂ or N₂, collides with them first. The other molecule, indicated as M, carries off some of the energy: 0 + 0₂ → 0₂* O₂ + MO₂ + M where * denotes a high-energy state. The net reaction, the sum of these two elementary reactions (after multiplying them by 2) and the reaction presented earlier, is 3 0₂ → 2 03. Some of the ozone is decomposed by ultraviolet radiation: UV,A<340 mm 0₂- 0+0₂ The oxygen atom produced in this step can react with oxygen molecules to produce more ozone, and so the ozone concentration Ozone hole area/(10 km²) 25 8 15 10 5 0 Seasonal variation in the mean area of the ozone hole over Antarctica for 2014. in the stratosphere normally remains constant, with seasonal variations. Because the decomposition of ozone absorbs ultraviolet radiation, ozone helps to shield the Earth from radiation damage. Warnings of the possibility that anthropogenic (human- generated) chemicals can threaten the ozone in the stratosphere began to appear in 1970 and 1971, when Paul Crutzen deter- mined experimentally that NO and NO₂ molecules catalyze the destruction of ozone. Nitrogen oxides are produced naturally in the troposphere by lightning and combustion in automobile and airplane engines. However, only N₂O is sufficiently unreactive to travel up to the stratosphere, where it is converted into NO₂. Mario Molina and Sherwood Rowland used Crutzen's work and other data in 1974 to build a model of the stratosphere that explained how chlorofluorocarbons could threaten the ozone layer.* In 1985, ozone levels over Antarctica were indeed found
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
1 2...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Emily Jackson (Social Security number 765-12-4326) and James Stewart (Social Security number 466-74-9932) are partners in a partnership that owns and operates a barber shop. The partnership's first...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Unfortunately, intellectual property law cannot protect the business everywhere. For example, there is a flood of cheap imitations of Japanese motorcycles on the Chinese market, and Honda Motor...
-
) A local firm employs 20 full-time professionals. The budgetedannual compensation per employee is $81,000. The budgeted averagechargeable time is 500 hours per client annually. All professionallab 2...
-
Here are inflation rates and stock market and Treasury bill returns between 1996 and 2000: a. What was the real return on the S&P 500 in each year? b. What was the average real return? c. What was...
-
The signal x c (t) = sin(2 (100)t) was sampled with sampling period T = 1/400 second to obtain a discrete-time signal x[n]. What is the resulting signal x[n]?
-
Visit the Transmission Versus Propagation Delay applet at the companion Web site. Among the rates, propagation delay, and packet sizes available, find a combination for which the sender finishes...
-
Forming, the second department in a three-department production process for Chula Vista Can Inc., received 15,000 units with a total cost of $45,000 from Blanking during the month of May. Production...
-
Calculate the required rate of return for Mudd Enterprises assuming that investors expect a 5.0% rate of inflation in the future. The real risk-free rate is 1.0%, and the market risk premium is 7.5%....
-
The contribution to the destruction of the ozone layer caused by high-flying aircraft has been attributed to the following mechanism: (a) Write the overall reaction. (b) Write the rate law for each...
-
Cyanomethane, commonly known as acetonitrile, CH 3 CN, is a toxic volatile liquid which is used as a solvent to purify steroids and to extract fatty acids from fish oils. Acetonitrile can be...
-
Suppose you see evidence that the stock market is efficient. Would that make you more or less likely to invest in stocks for your 401(k) retirement plan when you get your first job?
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
(Requires calculus) (i) Suppose in the Tobit model that x1 = log(z1), and this is the only place z1 appears in x. Shows that Where 1 is the coefficient on log(z1). (ii) If x1 = z1, and x2 = z21, show...
-
What are some of the possible sources of information about a company that could be used for determining the companys competitive stance?
-
Calculate the expectation value of the radius r at which you would find the electron if the H atom wave function is 100 (r).
-
Calculate the expectation value for the kinetic energy of the H atom with the electron in the 2s orbital. Compare your result with the total energy.
-
Ions with a single electron such as He + , Li 2+ , and Be 3+ are described by the H atom wave functions with Z/a 0 substituted for 1/a 0 , where Z is the nuclear charge. The 1s wave function becomes ...
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


