The solubility of benzoic acid, is (0.34 mathrm{~g} / 100 mathrm{~mL}) in water at (25^{circ} mathrm{C}) and
Question:
The solubility of benzoic acid,
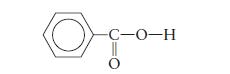
is \(0.34 \mathrm{~g} / 100 \mathrm{~mL}\) in water at \(25^{\circ} \mathrm{C}\) and \(10.0 \mathrm{~g} / 100 \mathrm{~mL}\) in benzene \(\left(\mathrm{C}_{6} \mathrm{H}_{6}ight)\) at \(25^{\circ} \mathrm{C}\). Rationalize this solubility behavior. For a \(1.0 \mathrm{~m}\) solution of benzoic acid in benzene, would the measured freezing-point depression be equal to, greater than, or less than \(5.12^{\circ} \mathrm{C}\) ? \(\left(K_{\mathrm{f}}=5.12^{\circ} \mathrm{C} \mathrm{kg} / \mathrm{mol}ight.\) for benzene.)
Transcribed Image Text:
-C-0-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To rationalize the solubility behavior of benzoic acid in water versus benzene we can turn to the pr...View the full answer

Answered By

Nicholas Maina
Throughout my tutoring journey, I've amassed a wealth of hands-on experience and honed a diverse set of skills that enable me to guide students towards mastering complex subjects. My proficiency as a tutor rests on several key pillars:
1. Subject Mastery:
With a comprehensive understanding of a wide range of subjects spanning mathematics, science, humanities, and more, I can adeptly explain intricate concepts and break them down into digestible chunks. My proficiency extends to offering real-world applications, ensuring students grasp the practical relevance of their studies.
2. Individualized Guidance:
Recognizing that every student learns differently, I tailor my approach to accommodate various learning styles and paces. Through personalized interactions, I identify a student's strengths and areas for improvement, allowing me to craft targeted lessons that foster a deeper understanding of the material.
3. Problem-Solving Facilitation:
I excel in guiding students through problem-solving processes and encouraging critical thinking and analytical skills. By walking learners through step-by-step solutions and addressing their questions in a coherent manner, I empower them to approach challenges with confidence.
4. Effective Communication:
My tutoring proficiency is founded on clear and concise communication. I have the ability to convey complex ideas in an accessible manner, fostering a strong student-tutor rapport that encourages open dialogue and fruitful discussions.
5. Adaptability and Patience:
Tutoring is a dynamic process, and I have cultivated adaptability and patience to cater to evolving learning needs. I remain patient through difficulties, adjusting my teaching methods as necessary to ensure that students overcome obstacles and achieve their goals.
6. Interactive Learning:
Interactive learning lies at the heart of my approach. By engaging students in discussions, brainstorming sessions, and interactive exercises, I foster a stimulating learning environment that encourages active participation and long-term retention.
7. Continuous Improvement:
My dedication to being an effective tutor is a journey of continuous improvement. I regularly seek feedback and stay updated on educational methodologies, integrating new insights to refine my tutoring techniques and provide an even more enriching learning experience.
In essence, my hands-on experience as a tutor equips me with the tools to facilitate comprehensive understanding, critical thinking, and academic success. I am committed to helping students realize their full potential and fostering a passion for lifelong learning.
4.90+
5+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In Exercises 51-54, a. determine the value that the function f approaches as the magnitude of x increases. Is f (x) greater than or less than this value when b. x is positive and large in magnitude...
-
The accompanying data are x = cost (cents per serving) and y = fiber content (grams per serving) for 18 high-fiber cereals rated by Consumer Reports (www .consumerreports.org/health). a. Compute and...
-
Which statement is correct? A) Tax credits reduce tax liability on a dollar-for-dollarbasis. B) Tax deductions reduce tax liability on a dollar-for-dollarbasis. C) The benefit of a tax credit depends...
-
The population of the world was about 5.3 billion in 1990. Birth rates in the 1990s ranged from 35 to 40 million per year and death rates ranged from 15 to 20 million per year. Lets assume that the...
-
A specific consumer group to which a product is designed to appeal Match the terms with the definitions. Some terms may not be used. a. company advertising b. custom c. economy d. industry...
-
7 La fase de establecimiento de metas en la etapa de planeacin del proceso de marketing estratgico fija objetivos cuantificados para usarlos en la fase de evaluacin. Qu puede hacer un gerente si los...
-
Using a table similar to that shown in Figure 3.6, calculate the product of the octal unsigned 6-bit integers 62 and 12 using the hardware described in Figure 3.3. You should show the contents of...
-
Hawkeye Networks is a U.S. corporation with $20 million of U.S. source income and no foreign-source income of its own. Hawkeye Networks has wholly owned subsidiaries in Korea and Singapore. The...
-
A sample containing \(0.0500 \mathrm{~mol}\) of \(\mathrm{Fe}_{2}\left(\mathrm{SO}_{4}ight)_{3}\) is dissolved in enough water to make \(1.00 \mathrm{~L}\) of solution. This solution contains...
-
The Tyndall effect is often used to distinguish between a colloidal suspension and a true solution. Explain.
-
Find each value. If applicable, give an approximation to four decimal places. log (387 23)
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
The model in Exercise 32 starting from (0.5, 1).
-
Why do CPA firms sometimes use a combination of positive and negative confirmations on the same audit?
-
The production of steel from iron ore is endothermic. To reduce the heat that must be supplied, engineers need to find the lowest temperature at which the desired reactions are spontaneous. Estimate...
-
By assuming that the lattice enthalpy of NaCl 2 is the same as that of MgCl 2 , use enthalpy arguments based on data in Appendix 2A, explain why NaCl 2 is an unlikely compound. 2A THERMODYNAMIC DATA...
-
The standard entropy of vaporization of acetone is approximately 85 J K 1 mol 1 at its boiling point. (a) Estimate the standard enthalpy of vaporization of acetone at its boiling point of 56.2 C....
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


