The structure below shows a hydrated d-metal ion. Draw the structure of the conjugate base of this
Question:
The structure below shows a hydrated d-metal ion. Draw the structure of the conjugate base of this complex.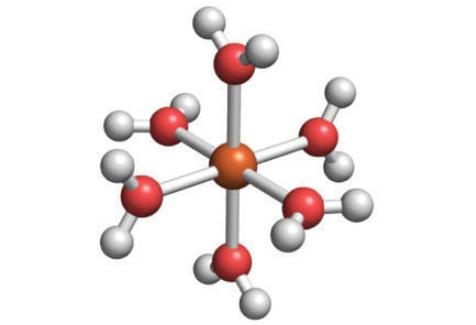
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The image youve provided shows a metal ion in orange surrounded by water molecules which are the lig...View the full answer

Answered By

Sultan Ghulam Dastgir
The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Vasovist is the tradename of a Gd(III) complex which was the first intravascular contrast agent (see Box 4.3) approved in the EU for use in magnetic resonance angiography. Interactions between the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The Delegator Instructions: You are a manager for a busy, medium-sized company. You have recently been ill, and the doctor has told you that you need to cut your workload in half. Your...
-
5.Construct a Histogram for the frequency distribution below containing record high temperatures for each of the 50 states. Class boundaries Freq 99.5-104.5 104.5-109.5 109.5-114.5 114.5-119.5...
-
Quality improvement, relevant costs, relevant revenues. TechnoPrint manufactures and sells 20,000 high-technology printing presses each year. The variable and fixed costs of rework and repair are as...
-
How many orders of the entire visible spectrum (400700 nm) can be produced by a grating of 500lines/mm?
-
Refer to your results from Exercise 1 in Chapter 1, in which effects on the speed of greyhounds were modelled. Obtain standard errors for the estimates of the constant and of the effect of age that...
-
City Car Wash, Inc., has current assets of $180 million; property, plant, and equipment of $300 million; and other assets totaling $110 million. Current liabilities are $105 million and long-term...
-
Lycan, Inc., has 7.1 percent coupon bonds on the market that have 9 years left to maturity. The bonds make annual payments and have a par value of $1,000. If the YTM on these bonds is 9.1 percent,...
-
PART-4 PART-5 PART-6 Jaguar Plastics Company has been operating for three years. At December 31 of last year, the accounting records reflected the following: Cash Investments (short-term) Accounts...
-
The autoprotolysis constant, K hw , for heavy water, D 2 O, at 25C is 1.35 * 10 15 . (a) Write the chemical equation for the autoprotolysis (more precisely, the autodeuterolysis, because a deuteron...
-
Draw the Lewis structure or symbol of each reactant, identify the Lewis acid and the Lewis base, and then draw the Lewis structure of the product (a complex) for the following Lewis acidbase...
-
Consider the following ER model for a course administration. Which statement is not correct? a. When mapping the ER relationship type teaches between Session and Teacher to the relational model, a...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
8 Two varieties of lettuce were grown for 16 days in a controlled environment. The following table shows the total dry weight (in grams) of the leaves of nine plants of the variety "Salad Bowl" and...
-
Refer to Exercise 8.S.I. Construct a scatterplot of the data. Does the appearance of the scatterplot indicate that the pairing was effective? Explain. Exercise 8.S.I. A volunteer working at an animal...
-
A hard-working horse can lift a 350. lb. weight 100. ft. in one minute. Assuming the horse generates energy to accomplish this work by metabolizing glucose: C 6 H 12 O 6 (s) + 6O 2 (g) 6CO 2 (g) +...
-
Under what conditions is the distribution of products in an ideal gas reactions system at equilibrium unaffected by an increase in the pressure?
-
Identify which of the following compounds is most activated toward electrophilic aromatic substitution. Which compound is least activated? Br NO2 NO2 OMe .
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...

Study smarter with the SolutionInn App


