The unit cell for a pure xenon fluoride compound is shown in the following diagram. a. What
Question:
The unit cell for a pure xenon fluoride compound is shown in the following diagram.
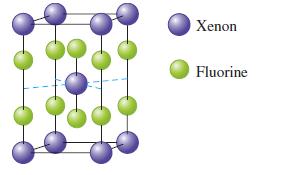
a. What is the formula of the compound?
b. The unit cell of \(\mathrm{XeF}_{2}\) has a height of \(702 \mathrm{pm}\), and the edge of the square base has a length of \(432 \mathrm{pm}\). Calculate the density of \(\mathrm{XeF}_{2}\).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





