Three unknown substances were tested in order to classify them. The following table shows the results of
Question:
Three unknown substances were tested in order to classify them. The following table shows the results of the tests. Use Table 3H.1 to classify substances A, B, and C as metallic, ionic, network, or molecular solids.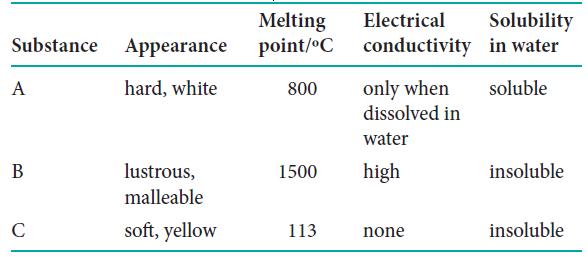
Transcribed Image Text:
Substance Appearance hard, white A B C lustrous, malleable soft, yellow Melting point/°C 800 Electrical Solubility conductivity in water soluble only when dissolved in water 1500 high 113. none insoluble insoluble
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Substance A i...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Three unknown substances were tested in order to classify them. The following table shows the results of the tests. Use Table 3H.1 to classify substances X, Y, and Z as metallic, ionic, network, or...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
The following table shows the returns and valuations of selected listed retailers. The table is followed by an extract of an article reporting on retailers' profit results. Discretionary retailers'...
-
The comparative balance sheet of Beets Industries, Inc. at December 31, 2013 and 2012, is as follows: An examination of the income statement and the accounting records revealed the following...
-
Larussa Inc. is preparing its annual budgets for the year ending December 31, 2011. Accounting assistants furnish the data shown below. An accounting assistant has prepared the detailed manufacturing...
-
Fill in the blank with an appropriate word, phrase, or symbol(s). A voting method that always satisfies the majority criterion and the monotonicity criterion, but may not satisfy any other criterion,...
-
and the Laspeyres index of part a on the same graph. Comment on the differences between the two indexes.
-
Mr. Robbinss detailed medical records indicated that a total of 20 labor hours were directly used in pro-viding his care. The cost of the labor was $ 380. It was expected before the beginning of the...
-
Exercise 10-14 (Static) Special offer pricing LO P7 Pardo Company produces a single product and has capacity to produce 120,000 units per month. Costs to produce its current monthly sales of 80,000...
-
Suppose that 200. mL of hydrogen chloride gas at 690. Torr and 20.C is dissolved in 100. mL of water. The solution is titrated to the stoichiometric point with 15.7 mL of a sodium hydroxide solution....
-
(a) A flask of volume 350. mL contains 0.1500 mol Ar at 24 C. What is the pressure of the gas in kilopascals? (b) You are told that 23.9 mg of bromine trifluoride exerts a pressure of 10.0 Torr at...
-
Had the securitized finance receivables been held on the balance sheet, Software Services ratio of liabilities to total capital would have been closest to: A. 73.0%. B. 74.8%. C. 80.4%. Michael...
-
Consider the following double loop and identify the dependence. Verify the dependence(s) by applying GCD test and Bounds test. Use the direction vector of (1, -1) corresponding to T on (5M) S. L1: do...
-
After discovering a vulnerability in the passwd utility, the Linux developers have decided that it is too dangerous to continue to run the utility as root (through setuid). Unfortunately, there's no...
-
Your supervisor comes to you and says, that as a company, you're facing multiple communication challenges. Since transitioning to a remote workforce, communication has been extremely poor....
-
You are developing an application that will be running on several hundred Amazon EC2 instances. The application on each instance will be required to reach out through a file system protocol...
-
What is the danger of mixing elements ("hybrid strategy") from both agile and lean SC strategies?
-
LeBlanc Inc. has always been the sole supplier of CAV, a raw material needed by Anderson Company. LeBlanc has steadily increased the price of CAV to $100 per pound. Badly in need of a second source,...
-
Southwestern Punch was made by Frutayuda, Inc. and sold in 12-ounce cans to benefit victims of Hurricane Zero. The mean number of ounces placed in a can by an automatic fill pump is 11.7 with a...
-
The freezing point of an aqueous solution is -2.79 C. a. Determine the boiling point of this solution. b. Determine the vapor pressure (in mm Hg) of this solution at 25 C (the vapor pressure of...
-
If the human eye has an osmotic pressure of 8.00 atm at 25 C, what concentration of solute particles in water will provide an isotonic eyedrop solution (a solution with equal osmotic pressure)?
-
At 25 C, the vapor in equilibrium with a solution containing carbon disulfide and acetonitrile has a total pressure of 263 torr and is 85.5 mole percent carbon disulfide. What is the mole fraction...
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


