Use Fig. 5B.2 to predict the phase of a sample of water under the following conditions: (a)
Question:
Use Fig. 5B.2 to predict the phase of a sample of water under the following conditions:
(a) 1 atm, 200°C;
(b) 100. atm, 50.0°C;
(c) 3 Torr, 10.0°C.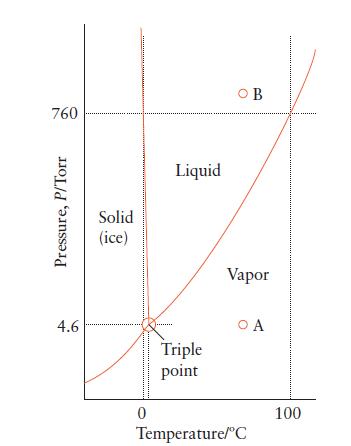
Transcribed Image Text:
760 Pressure, P/Torr 4.6 Solid (ice) 0 Liquid Triple point OB Vapor O A Temperature/C 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Va...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Find z 0 such that P (1.2 < z < z 0) = 0.8671. Find z 0 such that P ( z 0 < z < 2.5) = 0.7672. Find Z 0 such that the area between Z 0 and z = 0.5 is 0.2345 Find z0 such that P(z0 < z...
-
Arbitration, mediation, reference to a third person , an association tribunal, summary jury trial, rent-a-judge, minitrials and judicial triage, are all forms of what is known as?
-
The quantity of heat Q that changes the temperature T of a mass m of a substance is given by Q = cmT, where c is the specific heat capacity of the substance. For example, for H20, c = 1 cal/gC. And...
-
A clinical study is established to determine if the results of a screening stress test can be used as a predictor of the presence of heart disease. The study enrolls 100 participants who undergo a...
-
A 1.0-F capacitor is connected in parallel with a 2.0-F capacitor, and the combination is connected in series with a 6.0-F capacitor. What is the equivalent capacitance of this combination?
-
An uncharged 10-uF capacitor is charged by the current I(t)=10cos377t mA. Find (a) the expression for the voltage across the capacitor and (b) the expression for the power.
-
3. Pop Corporation sells inventory items for $500,000 to Son Corporation, its 80 percentowned subsidiary. The consolidated workpaper entry to eliminate the effect of this intercompany sale will...
-
Ladora Construction Company began operations on January 1, 2019, when it acquired $30,000 cash from the issuance of common stock. During the year, Ladora purchased $6,000 of direct raw materials and...
-
1) Bramble Corporation issued 4,000 of its common shares for $66,000. The company also incurred $1,700 of costs associated with issuing the shares. Prepare a single combined journal entry to record...
-
The Pilot Pen Company has decided to use 15 test markets to examine the sensitivity of demand for its new product to various prices, as shown in the following table. Advertising effort was identical...
-
Which would be the better solvent, water or benzene, for each of the following substances: (a) KCl; (b) CCl 4 ; (c) CH 3 COOH?
-
If you are an environmental scientist, you need to know the theoretical limits on the amount of oxygen that water can dissolve in order to monitor the capacity of natural waters to sustain life....
-
Use the accompanying diagram to answer ac. a. Indicate the efficient result on the graph. b. Illustrate the profits or losses from the efficient result in a. c. Show the average cost-pricing...
-
Problem 8-19 (Algo) Cash Budget; Income Statement; Balance Sheet [LO8-2, LO8-4, LO8-8, LO8-9, LO8- 10] Minden Company is a wholesale distributor of premium European chocolates. The company's balance...
-
Consider the unsteady flow of a fluid in the x direction through a control volume. The linear momentum of the fluid within the control volume is a function of time given by 200ti slug*ft/s, where t...
-
For a continuous uniform distribution with u = 0 and o = 1, the minimum is - V3 and the maximum is V3. For this continuous uniform distribution, find the probability of randomly selecting a value...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
a) List the ways in which data can be lost, adding some of your own. b) How does backup ensure availability?
-
H.J. Heinzs annual dividends were as follows: 1990 ..............$0.540 1991.............. 0.620 1992 .............. 0.700 1993.............. 0.780 1994 .............. 0.860 1995 .............. 0.940...
-
Show how a Wittig reaction can be used to prepare each of the following compounds. In each case, also show how the Wittig reagent would be prepared: (a) (b)
-
Choose a Grignard reagent and a ketone that can be used to produce each of the following compounds: (a) 3-methyl-3-pentanol (b) 1-ethylcyclohexanol (c) Triphenylmethanol (d) 5-phenyl-5-nonanol
-
You are working in a laboratory, and you are given the task of converting cyclopentene into 1,5 pentanediol. Your first thought is simply to perform an ozonolysis followed by reduction with LAH, but...
-
question 1- You borrow a simple loan of SR 500,000, interest rate is 20%, it matures in one year. what's the yied to maturity? question 2- calculate_i for One-Year Discount Bond with price(p) =...
-
Taste of Muscat is a reputed chain of restaurants operating in Oman. Assume You are working as a management accountant for this restaurant chain which is specialized in all types of Arabic food. Your...
-
Industry Current Year Minus 1 Current Year Minus 2 Company: Air Products and Chemicals, Inc. (APD) Stock Price: 306.72 USD Shares Outstanding: 220.89 M Financial Ratios Most Current Year Current...

Study smarter with the SolutionInn App


