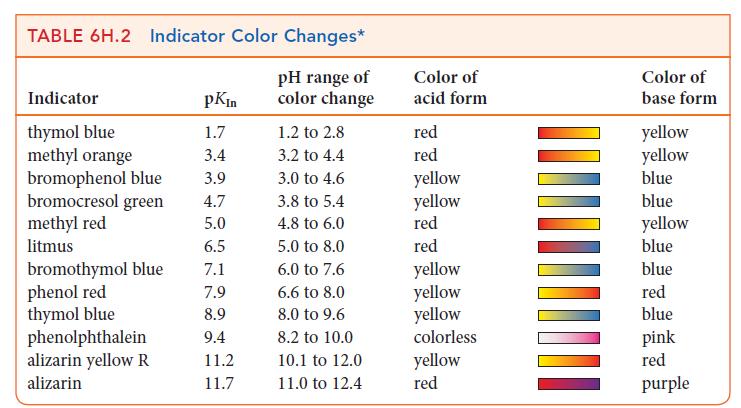
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.9 and 6H.14. Exercises
Question:
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.9 and 6H.14.
Exercises 6H.9
Benzoic acid, C6H5COOH, is used as a preservative in food and cosmetics because it is considered to be relatively safe.
Suppose you are studying benzoic acid and need to predict the pH of a benzoic acid solution during a titration. Calculate the pH at the stoichiometric point of the titration of 25.00 mL of 0.120 m C6H5COOH(aq) with 0.0230 m NaOH(aq).
Exercises 6H.14
Suppose that 30.0 mL of 0.12 m C6H5COOH(aq) is titrated with 0.20 m KOH(aq).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





