Use Table 7.2 to order the following from the strongest to the weakest acid. [mathrm{HClO}_{2}, mathrm{H}_{2} mathrm{O},
Question:
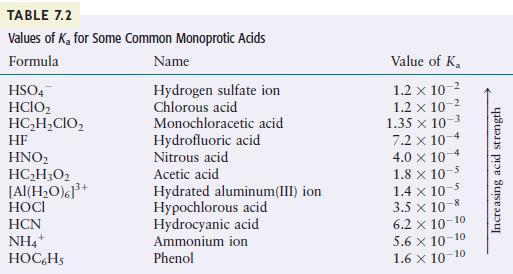
Use Table 7.2 to order the following from the strongest to the weakest acid.
\[\mathrm{HClO}_{2}, \mathrm{H}_{2} \mathrm{O}, \quad \mathrm{NH}_{4}^{+}, \quad \mathrm{HClO}_{4}\]
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10- 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10-5 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
The strength of an acid is often determined by its acid dissociation constant Ka The larger the Ka t...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 7 18, reduce each rational expression to lowest terms. x 2 + 4x + 4 x2 x - 4
-
(a) Show that the curve y = 23+3x-2 has no tangent line with slope 2. (b) Where does the normal line to the parabola y = z-r at the point (1,0) intersect the parabola a second time? Illustrate with a...
-
Use Table to order the following from the strongest to the weakest acid. HClO2, H2O, NH4+, HClO4 Table Formula Name Value of K 12 x 10-2 1.2 x 10-2 1.35 x 10-1 7.2 x 10-4 4.0 x 10-4 1.8 x 10-5 [ 1.4...
-
How do you identify the potential classes in a problem domain description?
-
With light falling normally on a transparent diffraction grating l0 mm wide, it was found that the components of the yellow line of sodium (589.0 and 589.6 rim) are resolved beginning with the fifth...
-
This question considers how the FX market will respond to changes in monetary policy. For these questions, define the exchange rate as Korean won per Japanese yen, EWON/. Use the FX and money market...
-
Learn about sociotechnical systems theory.(pp. 382385)
-
Calm Day reported the following income statement for the year ended December 31, 2019: Requirements 1. Compute Calm Day's inventory turnover rate for the year. (Round to two decimal places.) 2....
-
Determine the federal withholding for each employee. Lucien Laurin earns $2,190 semimonthly. He claims 4 allowances and is married. Employee: Laurin, Lucien Semimonthly Earnings $2,190.00 Federal...
-
You may need Table 7.2 to answer the following questions. a. Which is the stronger acid, \(\mathrm{H}_{2} \mathrm{SO}_{4}\) or \(\mathrm{H}_{2} \mathrm{O}\) ? b. Which is the stronger acid,...
-
For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and the conjugate acid. a. \(\mathrm{H}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{CO}_{3} ightleftharpoons...
-
You will be given $50 per day for meals when you travel, so you no longer need to provide receipts. Identify which job design technique is exemplified in each statement. A. Job simplification B. Job...
-
(b) A cylindrical storage tank with base area 90 m is being filled with water through an entry duct with cross-section area 250 cm, as shown in Figure 3. Concurrently, water is being extracted from...
-
Po A cylinder/piston arrangement contains 5 kg of water at 100 C with x= 20%. Initially the piston of mass m, 75 kg rests on a set of stops (see figure). The outside pressure is 100 kPa, and the area...
-
TABLE 2 Present Value of an Annuity of $1 n 123456 8% 9% 0.925926 0.917431 4 7 8 9 10 11 12 13 14 15 16 17 11.652296 10.837770 10.105895 9.446649 12.165669 11.274066 10.477260 9.763223 18 19...
-
There are 4 suits (heart, diamond, clover, and spade) in a 52-card deck, and each suit has 13 cards. Suppose your experiment is to draw one card from a deck and observe what suit it is. Express the...
-
Write isotopic symbol of zirconium and how many neutrons are present in one atom of this isotope
-
The demand for t-shirts is Q = 20 ( P/2, where Q is the number of t-shirts and P is their price. The private cost of producing t-shirts is C(Q) = Q2, with private marginal cost MCP = 2Q. Washing and...
-
Find the area of the surface generated by revolving the para- metric curve x = cos 1, y = sin? 1 (0 < I sa/2) about the y-axis.
-
Scientists often find it helpful to use very simple expressions to estimate the order of magnitude of a property without doing a detailed calculation. Treat a hydrogen atom as a one-dimensional box...
-
Arrange the elements in each of the following sets in order of decreasing atomic radius: (a) Sulfur, chlorine, silicon; (b) Cobalt, titanium, chromium; (c) Zinc, mercury, cadmium; (d) Antimony,...
-
Rainbows form when the wavelengths of sunlight are refracted (bent) through different angles. When sunlight passes through droplets of water, the shorter the wavelength of the light, the greater the...
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


