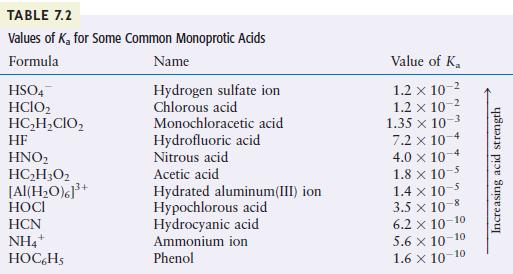
You may need Table 7.2 to answer the following questions. a. Which is the stronger acid, (mathrm{H}_{2}
Question:
You may need Table 7.2 to answer the following questions.
a. Which is the stronger acid, \(\mathrm{H}_{2} \mathrm{SO}_{4}\) or \(\mathrm{H}_{2} \mathrm{O}\) ?
b. Which is the stronger acid, \(\mathrm{H}_{2} \mathrm{O}\) or \(\mathrm{HOCl}\) ?
c. Which is the stronger acid, \(\mathrm{NH}_{4}{ }^{+}\)or \(\mathrm{HC}_{2} \mathrm{H}_{2} \mathrm{ClO}_{2}\) ?
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Based on the provided Table 72 which shows values of the acid dissociation constant K for some commo...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
You may need Table to answer the following questions. a. Which is the stronger acid, HCl or H2O? b. Which is the stronger acid, H2O or HNO2? c. Which is the stronger acid, HCN or HOC6H5? Table...
-
You have a job as the middle-level executive in a 250 bed hospital.The hospital is technically a "non-profit" hospital, but it is run as close to a "for profit" goal as possible.As a result of...
-
You may need Table to answer the following questions. a. Which is the stronger base, Cl2 or H2O? b. Which is the stronger base, H2O or NO2-? c. Which is the stronger base, CN2 or OC6H5-? Table...
-
Linda has d dollars in an account that pays 1.4% interest, compounded weekly. She withdraws w dollars. Express her first weeks interest algebraically.
-
A transparent diffraction grating of a quartz spectrograph is 25 mm wide and has 250 lines per millimeter. The focal length of an objective in whose focal plane a photographic plate is located is...
-
We can use the asset approach to both make predictions about how the market will react to current events and understand how important these events are to investors. Consider the behavior of the...
-
Explain how technology impacted people during the Industrial Revolutions.(pp. 382385)
-
Green rented an apartment from Stockton Realty. The three-story building had a washroom and clothesline on the roof for use by the tenants. The clothesline ran very near the skylight, and there was...
-
Question 07 Indicate whether each of the following accounts is an asset, a liability, or an owner's equity account and whether it has a normal debitor credit balance: (a) Accounts Receivable (b)...
-
You may need Table 7.2 to answer the following questions. a. Which is the stronger base, \(\mathrm{HSO}_{4}{ }^{-}\)or \(\mathrm{H}_{2} \mathrm{O}\) ? b. Which is the stronger base, \(\mathrm{H}_{2}...
-
Use Table 7.2 to order the following from the strongest to the weakest acid. \[\mathrm{HClO}_{2}, \mathrm{H}_{2} \mathrm{O}, \quad \mathrm{NH}_{4}^{+}, \quad \mathrm{HClO}_{4}\] TABLE 7.2 Values of K...
-
Why is it important that the planned image boost of an event relates to the destination positioning and brand?
-
Lucy is using a one-sample test based on a simple random sample of size = 24 to test the null hypothesis = 23.000 cm against the alternative hypothesis < 23.000 cm. The sample has mean 22.917 cm and...
-
A motorcyclist of mass 60 kg rides a bike of mass 40 kg. As she sets off from the lights, the forward force on the bike is 200N. Assuming the resultant force on the bike remains constant, calculate...
-
A load downward load P = 400 N is applied at B. It is supported by two truss members with member BA at an angle of 0 = 45 from horizontal and member BC at an 01 = angle of 02 25 from vertical....
-
Gross profit, defined as Net sales less Cost of products sold increased by $279 million in 2017 from 2016 and decreased by $2 million in 2016 from 2015. As a percent of sales, gross profit was 38.8%...
-
An electro-magnetic shield is to be made of galvanized steel with conductivity = 1.74 x 106 S/m, and magnetic permeability HR = 80. The thickness of cold rolled steel is in the following table. Gauge...
-
Welfare analysis can get complicated if there are multiple market failures. "The General Theory of the Second Best," by Lipsey and Lancaster (Review of Economic Studies 24 (1956-57): 11-32) argues...
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
(a) Using the particle-in-the-box model for the hydrogen atom and treating the atom as an electron in a one-dimensional box of length 150. pm, predict the wavelength of radiation emitted when the...
-
At the time that J . J. Thomson conducted his experiments on cathode rays, the nature of the electron was in doubt. Some considered it to be a form of radiation, like light; others believed the...
-
Which of the following increase when an electron in a lithium atom undergoes a transition from the 1s-orbital to a 2p-orbital? (a) Energy of the electron. (b) Value of n. (c) Value of l. (d) Radius...
-
Kelley Enterprises In October 1989, Pat Kelley.wus in his office, preparing the 1990 budget and contemplating the recent races of his business. Orders had been plentiful Lately that he though that...
-
The MegaMart Company began 2024 with inventory of 15,000 units at a cost of $6 per unit. During 2024, 55,000 units were purchased for $8.00 each. Sales for the year totaled 61,500 units leaving 8,500...
-
How much would Juanita's monthly premium be for a 10-year term insurance policy with a face value of $260,000, based on Table 19-1 and Table 19-2 (in $)? She turned 24 years old on her last birthday....

Study smarter with the SolutionInn App


