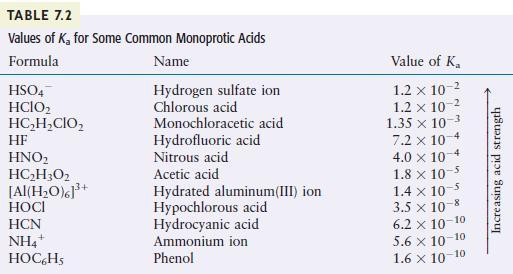
You may need Table 7.2 to answer the following questions. a. Which is the stronger base, (mathrm{HSO}_{4}{
Question:
You may need Table 7.2 to answer the following questions.
a. Which is the stronger base, \(\mathrm{HSO}_{4}{ }^{-}\)or \(\mathrm{H}_{2} \mathrm{O}\) ?
b. Which is the stronger base, \(\mathrm{H}_{2} \mathrm{O}\) or \(\mathrm{OCl}^{-}\)?
c. Which is the stronger base, \(\mathrm{NH}_{3}\) or \(\mathrm{C}_{2} \mathrm{H}_{2} \mathrm{ClO}_{2}{ }^{-}\)?
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10-5 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Of the two options provided in each question the stronger base is the one that is the conjugate base ...View the full answer

Answered By

Leah Muchiri
I am graduate in Bachelor of Actuarial Science and a certified accountant. I am also a prolific writer with six years experience in academic writing. My working principle are being timely and delivering 100% plagiarized free work. I usually present a precised solution to every work am assigned to do. Most of my student earn A++ GRADE using my precised and correct solutions.
4.90+
52+ Reviews
125+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
You have a job as the middle-level executive in a 250 bed hospital.The hospital is technically a "non-profit" hospital, but it is run as close to a "for profit" goal as possible.As a result of...
-
The accompanying table can be used to make paired comparisons of the desirability of salary, deferred compensation, and pensions as a function of a = current and future employer marginal tax rates, b...
-
Answer the following questions based on the information presented for Cloud 9 in Appendix B of this book and in the current and earlier chapters. You should also consider your answers to the case...
-
A palindrome is a string that is the same backward as it is forward. For example,tot and otto are rather short palindromes. Write a program that lets a user enter a string and that passes to a bool...
-
The ultimate resolving power λ/δλ of the spectrograph's trihedral prism is determined by diffraction of light at the prism edges (as in the case of a slit). When the prism is...
-
Use the table that follows to answer this question. Treat the country listed as the home country, and the United States as the foreign country. Suppose the cost of the market basket in the United...
-
Understand how computing and telecommunication technologies have evolved.(pp. 382385)
-
On January 1, 2014, the ledger of Werth Company contains the following liability accounts. Accounts Payable ......... $35,000 Sales Taxes Payable ........ 5,000 Unearned Service Revenue ..... 12,000...
-
Eaton Company manufactures wheel rims. The company produces two wheel rim models: standard and deluxe. For 2019, Eaton's managers have decided to use the same indirect manufacturing costs per wheel...
-
Classify each of the following as a strong acid, weak acid, strong base, or weak base in aqueous solution. a. \(\mathrm{HNO}_{2}\) b. \(\mathrm{HNO}_{3}\) c. \(\mathrm{CH}_{3} \mathrm{NH}_{2}\) d....
-
You may need Table 7.2 to answer the following questions. a. Which is the stronger acid, \(\mathrm{H}_{2} \mathrm{SO}_{4}\) or \(\mathrm{H}_{2} \mathrm{O}\) ? b. Which is the stronger acid,...
-
Light from two laser pointers with wavelengths 630 nm (red light) and 470 m (blue light) is incident on a diffraction grating with a slit spacing of 10 mm, and the diffracted light illuminates a...
-
Consider a rigid body B with center of mass point B*. A set of coordinate axes is chosen centered at B* and defined by mutually orthogonal unit vectors 61, 62, 63 which are fixed in B. The rigid body...
-
Let A be the matrix 1 0 2 4 1 -6 = 7-4 7 -5 3 (a) (2 points) What must a and b be in order to define the linear transformation T: RR by T(x) = Ax. (b) (3 points) What is the image of the vector 2] 1...
-
write a title Understanding the roots of modern educational practices can provide valuable insights into their effectiveness and potential for improvement. One such root influencing contemporary...
-
State the limit for each of the following using the graph. -6. -5 + -3- 3 -2 -2 0 2 -2- w. 3 4
-
Great Eastern Credit Union (GECU) has two operating departments (Branches and Electronic) and three service departments (Processing, Administration, and Maintenance). During July, the following costs...
-
A builder proposes a skyscraper that would block sunlight to the neighboring houses. The building would have net benefits to the builder of $100,000. The neighbors, who use some solar heating, would...
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
Mineralogists and geologists often need to identify the relative sizes of atoms to judge whether one mineral might be modified by the inclusion of alien ions. For example, the different colors of...
-
Astronomers are often very interested in the temperatures of stars (including the Sun) because that gives a clue to the stars size, composition, and age. The maximum intensity of solar radiation...
-
Lines in the Balmer series of the hydrogen spectrum are observed at 656.3, 486.1, 434.0, and 410.2 nm. What is the wavelength of the next line in the series?
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...
-
Which of the following are elements of a bootstrappable business model? Indicate ALL that apply. Large up-front capital investment Recurring revenue stream Long sales cycles Word of mouth advertising
-
Hooligan Adventure Supply produces and sells various outdoor equipment. The Molding and Assembly production departments are supported by the Personnel and Maintenance departments. Personnel costs are...

Study smarter with the SolutionInn App


