Using only the periodic table, predict the most stable ion for (mathrm{Na}, mathrm{Mg}, mathrm{Al}, mathrm{S}, mathrm{Cl}, mathrm{K},
Question:
Using only the periodic table, predict the most stable ion for \(\mathrm{Na}, \mathrm{Mg}, \mathrm{Al}, \mathrm{S}, \mathrm{Cl}, \mathrm{K}, \mathrm{Ca}\), and \(\mathrm{Ga}\). Arrange these from largest to smallest radius and explain why the radius varies as it does. Compare your predictions with Fig. 13.8.
Figure 13.8
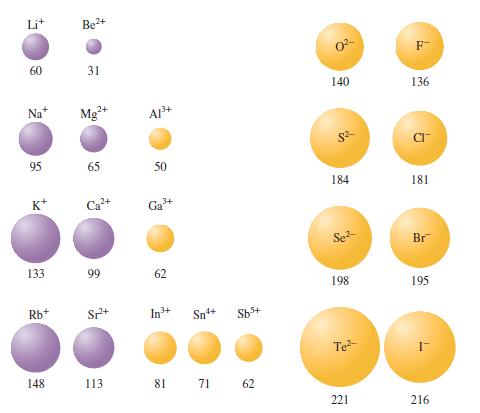
Transcribed Image Text:
60 Na* 95 K* 133 Rb+ Be+ 31 Mg+ 65 A1+ Sp+ 50 3+ Ga 99 62 In+ Sn4+ Sb+ 148 113 81 71 62 0- 140 $2 184 Se- 198 Te- 221 F- 136 CI- 181 Br 195 216
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Na Sodium Predicted most stable ion Na loses one electron to achieve a noble gas configuration Larger radius than Na Already provided Explanation The ...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the ionic compounds KF, NaCl, NaBr, and LiCl. (a) Use ionic radii (Figure 7.8) to estimate the cationanion distance for each compound. (b) Based on your answer to part (a), arrange these...
-
Graph f(x) - x, g(x) x +3 and (x) x5. Calculate the derivatives of f, g and h.
-
Using only the periodic table as your guide, select the most electronegative atom in each of the following sets: (a) Na, Mg, K, Ca; (b) P, S, As, Se; (c) Be, B, C, Si; (d) Zn, Ge, Ga, As.
-
Our model of pollution in this chapter assumed that emissions are a pure private bad, and that people have no ability to protect themselves from the adverse consequences of exposure. In reality,...
-
A particle of mass m is at r4est at the end of a spring (force constant = k) hanging from a fixed support. At t = 0, a constant downward force F is applied to the mass and acts for time t0. Show...
-
Baba Furniture Company employs four carpenters for 10 days to assemble tables and chairs. It takes 2 person-hours to assemble a table and .5 person-hour to assemble a chair. Customers usually buy one...
-
1 Below are some of the countries in which Starbucks operates. If Hofstedes analysis is accurate, what may be the implications for managing the business in each of those countries, and for a manager...
-
Following are selected accounts for Mergaronite Company and Hill, Inc., as of December 31, 2013. Several of Mergaronites accounts have been omitted. Credit balances are indicated by parentheses....
-
This problem consists of three independent parts relating to Companies X, Y, and Z: Company X invested $37,368 at the beginning of Year 1. The amount on deposit earned interest at 10% per year. The...
-
Which has the greater bond lengths: \(\mathrm{NO}_{2}{ }^{-}\)or \(\mathrm{NO}_{3}{ }^{-}\)? \(\mathrm{Ex}-\) plain.
-
Predict the trend in radius of the following ions: Be 2+ , Mg 2+ , Ca 2+ , and Sr 2+ .
-
Figure P15.72 shows an audio amplifier using two identical op-amps connected in a bridge configuration. (a) Derive the expression for the voltage gain \(A_{v}=v_{L} / v_{I}\). (b) Design the system...
-
1. Do you think that the NFL and franchise owners are meeting their obligations to employee health and safety? 2. Do you think that the NFL's and owners' responsibilities in terms of player safety...
-
Explain the term \'management\'. Also, explain briefly mission functions of management. ( b ) What are the different types of plant layout? Explain any two with neat sketches.
-
Suppose that you are considering an investment product that promises to pay $ 2 , 0 0 0 at the end of each year for the next five years. Assume that a discount rate of 1 2 % is applicable to similar...
-
Leadership Philosophy: Democratic and Transformational leadership In 700+ words ,explain how the leadership philosophy might impact an organization and how it would be beneficial.Identify what are...
-
performance and participation. The employee requirement that is met is status and recognition. The performance result is awakened drives. This model is dependent on leadership strive. It gives a...
-
Consider an ideal steam regenerative Rankine cycle with two feedwater heaters, one closed and one open. Steam enters the turbine at 10 MPa and 600°C and exhausts to the condenser at 10 kPa. Steam...
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s)| Fe+ (aq), Fe+ (aq)||Ag* (aq) Ag(s) (b) U(s) U+ aq||V+ (aq) V(s) 2+ (c) Sn(s) Sn+ (aq)||Sn4+ (aq),Sn+ (aq)|Pt(s)...
-
The molar solubility of silver sulfite, Ag 2 SO 3 , is 1.55 * 10 5 mol L 1 . What is the K sp of silver sulfite?
-
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: 2+ (a) Zn(s) Zn+ (aq)|| Au+ (aq)| Au(s) 3+ (b) Fe(s) | Fe+ (aq) || Fe+ (aq) |Fe(s)...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


