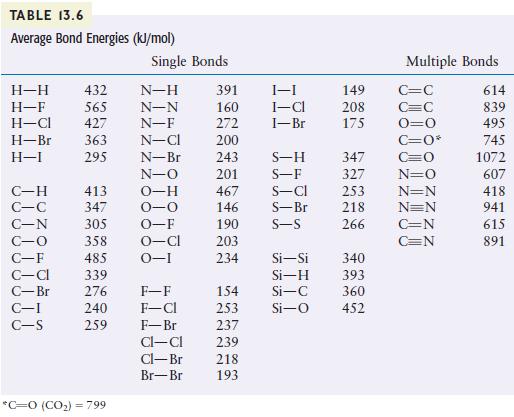
Using the bond energies listed in Table 13.6, calculate H for the reaction of methane with chlorine
Question:
Using the bond energies listed in Table 13.6, calculate ΔH for the reaction of methane with chlorine and fluorine to give Freon-12 (CF2Cl2).
![]()
Transcribed Image Text:
CH(g) + 2Cl(g) + 2F(g) CFCl(g) + 2HF(g) + 2HCl(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The idea here is to break the bonds in the reactants to give indi vidual atoms and then assemble the...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the average CH bond enthalpy in methane using the data tables. Calculate the percent error in equating the average CH bond energy in Table 4.3 with the bond enthalpy. Table 4.3 Selected...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Q3. Modulus of rupture of concrete is a measure of a) flexural tensile strength b) direct tensile strength c) compressive strength d) split tensile strength Q4. For a longitudinal reinforcing bar in...
-
(a) For what nonzero values of does the function y = sin kt satisfy the differential equation yn + 9y = 0? (b) For those values of k, verify that every member of the family of functions y = A sin kt...
-
Ramona Company uses an imprest petty cash system. The fund was established on March 1 with a balance of \(\$ 100\). During March the following petty cash receipts were found Prepare journal entries...
-
True or False. A value of the correlation coefficient near LO9 1 or near -1 implies a causal relationship between x and y.
-
On August 1, Rantoul Stores Inc. is considering leasing a building and purchasing the necessary equipment to operate a retail store. Alternatively, the company could use the funds to invest in...
-
a) Journal Entries Date Account Titles and Explanation Debit Credit Feb. 1 Investments in CBF Common Stock $39,650 Cash $39,650 (To record the purchase of 650 shares by cash) Mar. 1 Investments in...
-
Give the Lewis structure for each of the following. a. HF b. N 2 c. NH 3 d. CH 4 e. CF 4 f. NO +
-
Choose the largest ion in each of the following groups. a. Li + , Na + , K + , Rb + , Cs + b. Ba 2+ , Cs + , I , Te 2
-
A person stole a car from a car rental agency. The police spotted the car and gave chase. The thief hit another car, seriously injuring that driver. The person who stole the car fled and was not...
-
According to the College Board website, the scores on the math part of the SAT (SAT-M) in a certain year had a mean of 507 and a standard deviation of 111. Assume that SAT scores follow a normal...
-
Pay and incentive programs are being used both for knowledge workers and in non-knowledge worker occupations. In every industry, from restaurants to construction and low-tech manufacturing, companies...
-
Closet International invested in an equipment in 2019 with an initial cost of $598,000. It falls under asset class 8 with a CCA rate of 20%. The equipment was sold in 2021 for $260,000. Calculate the...
-
Question 4 (30 Marks) A 12-ply Kevlar/Epoxy composite beam with layup [0/90 / 0 1s is loaded in 3-point bending, as shown in Figure Q4. The beam has a length, L of 100mm, a width, b of 25mm and a...
-
Scenario: You have been working in a community service sector for two years. However, you always find evaluating your own performance challenging. Your Supervisor has also identified that you do not...
-
Suppose that the size of the first cell is 5.0 L and the second is 12.0 L. Follow these steps to derive the equations for chemical exchange between two adjacent cells of different size. Suppose the...
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Identify the hybrid orbitals used by the atom in boldface red type in each of the following molecules: (a) H 2 C C CH 2 ; (b) H 3 C C H 3 ; (c) CH 3 N N N; (d) CH 3 C O OH.
-
(a) Draw the molecular orbital energy-level diagram for N 2 and label the energy levels according to the type of orbitals from which they are made, whether they are - or -orbitals, and whether they...
-
Draw the Lewis structure for each of the following molecules or ions and give the number of electrons about the central atom: (a) SF 6 ; (b) XeF 2 ; (c) AsF 6 ; (d) TeCl 4 .
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


