Which of the following isomers of diaminobenzene can form chelating complexes? Explain your reasoning. (a) NH NH,
Question:
Which of the following isomers of diaminobenzene can form chelating complexes? Explain your reasoning.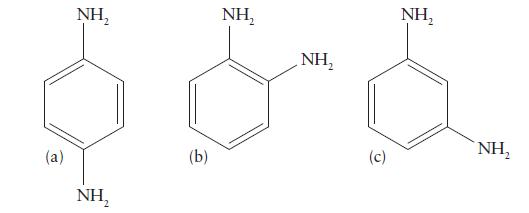
Transcribed Image Text:
(a) NH₂ NH, (b) NH₂ NH₂ (c) NH₂ NH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Only the molecule b can function as ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In which of the following complexes are geometric isomers possible? If isomers are possible, draw their structures and label them as cis or trans, or as fac or mer. (a) [Co(H 2 O) 4 Cl 2 ] + (b)...
-
1. Hannah is applying for a life policy on her girlfriend Sarahs life. The policy is $500,000 and carries a large premium. Hannah is the main earner, so she is concerned about not being able to pay...
-
The multiple choice questions presented in Question 2 above are low context. You should be able to answer them simply by a careful reading of the chapter. These questions, on the other hand, are high...
-
Find the struggles faced by business (printed t-shirts) with suppliers? Explain what would be the communication plan for each stakeholder and how you'll implement the decision you have taken?
-
Assume you have the following jobs to execute with one processor: Suppose a system uses RR scheduling with a quantum of 15. a. Create a Gantt chart illustrating the execution of these processes. b....
-
A deep drawing operation is performed in which the inside of the cylindrical cup has a diameter = 4.0 inches and a height = 2.5 inches. The stock thickness = 1/8 inch, and the starting blank diameter...
-
A rainbow is formed by refraction of light as it passes through a water droplet. In the ray diagram in Figure 24.49B, which of the following statements best explains the origin of a rainbow? Explain...
-
A diffraction grating has 4 200 rulings/cm. On a screen 2.00 m from the grating, it is found that for a particular order m, the maxima corresponding to two closely spaced wavelengths of sodium (589.0...
-
Wright Corporations contribution format income statement for at mothpears below Sales Less varaule expenses Contribution margin bud expenses Nut not $45,000 2000 14.000 12.00 550 There was no...
-
Solutions of the [V(OH 2 ) 6 ] 2+ ion are lilac in color and absorb light of wavelength 806 nm. What is the ligand field splitting in the complex in kilojoules per mole?
-
What is the oxidation number of (a) V in VO 2+ ; (b) Zn in [Zn(OH) 4 ] 2 ?
-
Assume that you own and operate a small business and that you have decided to expand with the help of general partners. Required 1. What details should you and your future partners specify in the...
-
Business Solutions's second-quarter 2022 fixed budget performance report for its computer furniture operations follows. The $175,750 budgeted expenses include $126,000 in variable expenses for desks...
-
Problem 2 (Numerical Integration) Using switch Statement and functions, write a single code to compute the following integral. 0 10 x +4 dx case 1: RECTANGULAR () // Rectangular rule case 2:...
-
Do you believe the elasticity of illicit narcotics is inelastic and if legalized demand will not increase? Do you also believe that many of society's social ills associated with drugs will ease not...
-
Stockstone Limited makes electric kettles that they currently sell at 13 each. The management believes that the company's equipment could currently produce up to 70,000 units of electric kettles per...
-
Jane Smith has worked for the Widgets, Weezles, and Warblers Corporation for the past 25 years. At a recent "Town Hall" meeting, Jane asked two members of the executive leadership team about their...
-
Consider the simple regression model with classical measurement error, y = (0 + (0x* + u, where we have m measures on x*. Write these as zh - x* + eh, h - 1, .... m. Assume that x* is uncorrelated...
-
1. Use these cost, revenue, and probability estimates along with the decision tree to identify the best decision strategy for Trendy's Pies. 2. Suppose that Trendy is concerned about her probability...
-
Although the vibrational degrees of freedom are generally not in the high-T limit, is the vibrational partition function evaluated by discrete summation?
-
What is the form of the total vibrational partition function for a polyatomic molecule?
-
How does the presence of degeneracy affect the form of the total vibrational partition function?
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...

Study smarter with the SolutionInn App


