Write balanced half-reactions and devise a galvanic cell (write a cell diagram) to study each of the
Question:
Write balanced half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: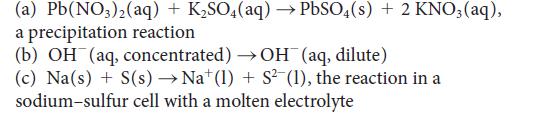
Transcribed Image Text:
(a) Pb(NO3)2(aq) + K₂SO4(aq) → PbSO4(s) + 2 KNO3(aq), a precipitation reaction (b) OH(aq, concentrated) →OH(aq, dilute) (c) Na(s) + S(s) → Nat (1) + S²(1), the reaction in a sodium-sulfur cell with a molten electrolyte
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a PbNO32aq K2SO4aq PbSOs 2 KNO3aq This is a precipitation reaction The halfreactions involved are th...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) AgBr(s) Ag (aq) + Br (aq), a solubility equilibrium (b) H(aq) + OH (aq) HO(1),...
-
(a) Write balanced half-reactions for the redox reaction between sodium perchlorate and copper (I) nitrate in an acidic solution. (b) Write the balanced equation for the cell reaction and devise a...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Since its 100% acquisition of Dancer Corporation stock on December 31, 2012, Jones Corporation has maintained its investment under the equity method. However, due to Dancer's earning potential, the...
-
Payback and NPV methods, no income taxes. (CMA, adapted) Andrews Construction is analyzing its capital expenditure proposals for the purchase of equipment in the coming year. The capital budget is...
-
An efficiency expert wishes to determine the average time that it takes to drill three holes in a certain metal clamp. How large a sample will he need to be 95% confident that his sample mean will be...
-
Suppose we wish to test for difference in population means among three groups. a. Explain why it is not sufficient to simply look at the differences among the sample means, without taking into...
-
Taos Company's record of transactions concerning part X for the month of April was as follows. Instructions (a) Compute the inventory at April 30 on each of the following bases. Assume that perpetual...
-
A direct quote of $1.9887 dollars to buy one U.K. pound corresponds to an indirect quote of .9887 pounds per one dollar. True False
-
Suppose an investment project costs $100,000 to start in period 0, and we know of the dividends it will return with certainty. The project returns dividends of $0 in period 0, $20,000 in period 1,...
-
Calculate the molar solubility of each substance in its respective solution: (a) Silver iodide in 0.020 m NaI(aq); (b) Calcium carbonate in 2.3 * 10 4 m Na 2 CO 3 (aq); (c) Lead(II) fluoride in 0.21...
-
Iron(II) hydrogen phosphite, FeHPO 3 , is oxidized by hypochlorite ions in basic solution. The products are chloride ions, phosphate ions, and iron(III) hydroxide. Write the balanced equation for...
-
For the following data, SS(within) = 116 and SS(total) = 338.769. (a) Find SS(between). (b) Compute MS(between), MS(within), and spooled. SAMPLE 31 30 39 34 26 45 39 35 39 32 29 37 30
-
Jimmy Joe-Bob Hicky is the district commander for the mostly-rural Spud Valley highway patrol district in western Idaho. Hes attempting to assign highway patrol cars to different road segments in his...
-
Its important to have a holistic view of all the businesses combined and ensure that the desired levels of risk management and return generation are being pursued. Agree or disagree
-
(3pts each) During a trip to a casino, Adam Horovitz plays his favorite casino game 10 times. Each time he plays, he has a 41% chance of winning. Assume plays of the game are independent. a. What is...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Statement of financial position as at 31 December 2014 ASSETS Non-current assets Property, plant and equipment Delivery van at cost 12,000 Depreciation (2,500) 9,500 Current assets Inventories...
-
Calculate the standard deviation, ÏY, of the random variable Y from Exercise 3.5.4. Exercise 3.5.4 Consider a population of the fruit-fly Drosophila melanogaster in which 30% of the individuals...
-
suppose a nickel-contaminated soil 15 cm deep contained 800 mg/kg Ni, Vegetation was planted to remove the nickel by phytoremediation. The above-ground plant parts average 1% Ni on a dry-weight bas...
-
Nitriles undergo alkylation at the α position much like ketones undergo alkylation at the α position. The α position of the nitrile is first deprotonated to...
-
Identify the Michael donor and Michael acceptor that could be used to prepare each of the following compounds via a Michael addition. (a) (b) (c) (d) (e) OEt N
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...

Study smarter with the SolutionInn App


