Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic
Question:
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: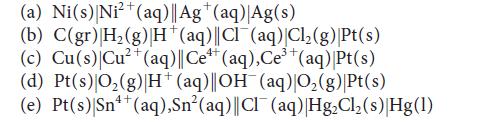
Transcribed Image Text:
(a) Ni(s) Ni²+ (aq)|| Ag+(aq) Ag(s) (b) C(gr) |H₂(g) H*(aq)||Cl¯(aq)|Cl₂(g)|Pt(s) (c) Cu(s) Cu²+ (aq)||Ce+ (aq), Ce³+ (aq) |Pt(s) (d) Pt(s)|O₂(g) |H+ (aq)||OH(aq)|O₂(g)|Pt(s) (e) Pt(s)|Sn++ (aq),Sn²(aq)||Cl¯ (aq) |Hg₂Cl₂(s) Hg(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a anode Nis Ni aq 2 e cathode Ag aq e Ags overall 2 Ag aq Nis 2 Ags Ni aq b anode H...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Explain how the following Arduino codes affect the motion of the motor. Which direction is the motor moving? Why the map commands are required? (6%) duty1 = 40; duty2 = 60; dutyl-map...
-
Write the half reactions for the electrolysis of the elements listed in Exercise 3.
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
Let z(k) denote the k-year continuously compounded zero-coupon yield for the current term structure. You are given that z(1) = 0.035, z(2) = 0.041, z(3) = 0.045, z(4) = 0.049, z(5) = 0.051, z(6) =...
-
Weighted-average method, assigning costs (continuation of 18-17). For the data in Exercise 18-17, summarize total costs to account for, calculate the cost per equivalent unit for direct materials and...
-
A study of the amount of rainfall and the quantity of air pollution removed produced the following data:(a) Find the equation of the regression line to predict, the particulate removed from the...
-
Discuss the common sources of risk on IT projects and suggestions for managing them. Which suggestions do you find most useful? Which do you feel would not work in your organization? Why? LO.1
-
Summarize the authors recommendations MINI CASE Overall, in the light of this study, we would come out strongly in favor of post-project reviews (provided you do not call them post mortems). We could...
-
Donald Biden is a portfolio manager who likes to invest his client's money to invest in highly speculative instruments. Donald is contemplating the purchase of 40,000 shares of Shakee Corp. common...
-
The following multiple linear regression depicts the cost structure of ABC Corporation: TC=10+2Q+1.5w+2r Where: TC: Total cost (S); Q: Quantity produced (Kg); w: Wage ($/hour); and r: Interest rates...
-
A careless laboratory technician wants to prepare 200.0 mL of a 0.025 m HCl(aq) solution but uses a volumetric flask of volume 250.0 mL by mistake. (a) What would the pH of the desired solution have...
-
The concentration of CrO 4 2 in a saturated Tl 2 CrO 4 solution is 6.3 * 10 5 mol L 1 . What is the K sp of Tl 2 CrO 4 ?
-
It was shown in Example 21.11 (Section 21.5) that the electric field due to an infinite line of charge is perpendicular to the line and has magnitude E = /2є0r. Consider an imaginary cylinder...
-
You will be creating a Performance Improvement Plan to address an employee in the attached case study (see below). This is a scenario you may encounter in your future HR profession, so this...
-
For this prompt, consider your academic goals, including (but not limited to) such topics as how you plan to manage your time to fit in your studies; how you will build your skills, as needed; how...
-
1. An introduction of you as a leader (whether or not you see yourself as a leader, whether or not you like being a leader, what kinds of leadership roles you have had, etc.). 2. Summarize your...
-
Briefly, describe the firm in terms of the following items. a. Size in terms of market capitalization, annual revenue, number of employees, location(s). b. Discuss the financial position of the firm....
-
HealthyLife (HL) is a publicly-traded company in the Food Manufacturing Industry. HealthyLife has been around since the 1970s, and is mainly focused on the production and wholesale of "organic and...
-
Refer to the IQ distribution of Exercise 4.S.12. Suppose five children are to be chosen at random from the population. Find the probability that exactly one of them will have an IQ score of 80 or...
-
Reichenbach Co., organized in 2018, has set up a single account for all intangible assets. The following summary discloses the debit entries that have been recorded during 2018 and 2019. Instructions...
-
Which of the following functions are eigenfunctions of the operator B if B f (x) = d 2 f (x) / dx 2 : x 2 , cos x, e 3ix ? State the eigenvalue if applicable.
-
Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? a. b. c. ei(7x+y) ax? x? + 2y? +...
-
Because d -d cos (nx / d) cos (mx / dx) = 0, m n, the functions cos (n x/d) for n = 1, 2, 3, . . . form an orthogonal set in the interval (d, d). What constant must these functions be multiplied by...
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....
-
What is the definition of substantially appreciated inventory? A. Inventory with a FMV greater than its basis B. Inventory and unrealized receivables with a FMV greater than their basis C. Inventory...

Study smarter with the SolutionInn App


