You are working in a plant that produces copper for use in electrical circuits by electrolysis of
Question:
You are working in a plant that produces copper for use in electrical circuits by electrolysis of acidic solutions of CuSO4(aq). A customer has placed an order for a small quantity of high-purity copper, and you need to tell the customer how long it will take to produce the metal. How many hours are required to plate 25.00 g of copper metal from 1.00 m CuSO4(aq) by using a current of 3.00 A?
PLAN Use the second procedure in Toolbox 6O.1.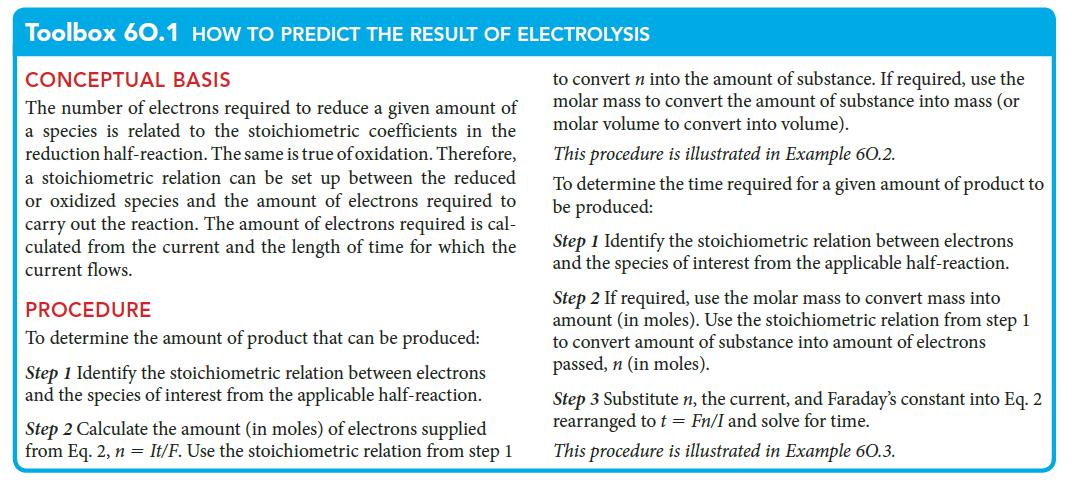
Transcribed Image Text:
Toolbox 60.1 HOW TO PREDICT THE RESULT OF ELECTROLYSIS CONCEPTUAL BASIS The number of electrons required to reduce a given amount of a species is related to the stoichiometric coefficients in the reduction half-reaction. The same is true of oxidation. Therefore, a stoichiometric relation can be set up between the reduced or oxidized species and the amount of electrons required to carry out the reaction. The amount of electrons required is cal- culated from the current and the length of time for which the current flows. PROCEDURE To determine the amount of product that can be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 Calculate the amount (in moles) of electrons supplied from Eq. 2, n = It/F. Use the stoichiometric relation from step 1 to convert n into the amount of substance. If required, use the molar mass to convert the amount of substance into mass (or molar volume to convert into volume). This procedure is illustrated in Example 60.2. To determine the time required for a given amount of product to be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 If required, use the molar mass to convert mass into amount (in moles). Use the stoichiometric relation from step 1 to convert amount of substance into amount of electrons passed, n (in moles). Step 3 Substitute n, the current, and Faraday's constant into Eq. 2 rearranged to t = Fn/I and solve for time. This procedure is illustrated in Example 60.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Step 1 Find the stoichiometric relation between electrons and the species of interest ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
1. You will be implementing the Generalized Arc Consistency (GAC) algorithm described in Chapter 4 of the textbook. In particular, you will be implementing the while loop shown in Figure 4.3:...
-
Elysian Fields, Inc., uses a maximum payback period of 6 years and currently must choose between two mutually exclusive projects. Project Hydrogen requires ar initial outlay of $25,000; project...
-
A customer has placed an order for 30,000 first-aid kits that must be delivered within 20 business days. The kits are to be assembled on the four-workstation line shown below and in kanban quantities...
-
Use hand calculations to fit the multiple linear regression model 1 y = β0 + β1x1 + β2x2 to the data set in DS 13.6.2. (a) Write down the vector of observed values...
-
Make versus buy, activity-based costing opportunity costs. (N. Melumad and S. Reichelstein, adapted) The Ace Company produces bicycles. This years expected production is 10,000 units. Currently, Ace...
-
The IQs of 600 applicants of a certain college are approximately normally distributed with a mean of 115 and a standard deviation of 12. If the college requires an IQ of at least 95, how many of...
-
Research risk management software. Are many products available? What are the main advantages of using them in managing projects? What are the main disadvantages? Write a short paper to discuss your...
-
On January 1, 2015, Harter Company had Accounts Receivable $139,000, Notes Receivable $25,000, and Allowance for Doubtful Accounts $13,200. The note receivable is from Willingham Company. It is a...
-
f on Your manager has told you to increase the discount rate you have been using to evaluate projects. With the new discount rate, you may need to update project evaluation using the following tools...
-
Following are separate financial statements of Michael Company and Aaron Company as of December 31, 2013 (credit balances indicated by parentheses). Michael acquired all of Aarons outstanding voting...
-
Muscles produce lactic acid during exercise. Calculate the pH, pOH, and percentage deprotonation of the following aqueous solutions of lactic acid, CH 3 CH(OH)COOH: (a) 0.11 m; (b) 3.7 * 10 3 m; (c)...
-
Wine is a complex mixture of over 1000 compounds, many at very low concentrations. A large number of flavorful components are derivatives of phenol, C 6 H 5 OH, a weak acid. If you are an enologist...
-
To find out if wealthier women tend to have Cesarean sections (C-sections) more often than poorer women, a sample of recent mothers was classified by socioeconomic status and whether they delivered...
-
D manufacturing Company uses a process cost system. In the second department, Department X, spoiled units occur when units are 70% complete. Direct Materials are added at the end of the process....
-
This week, I read Pat Friman's 2021 article, "There Is no Such Thing As a Bad Boy: The Circumstances View of Problem Behavior," on adopting The Circumstances View when working with clients. In this...
-
Download the labour force statistic for Australia from the ABS website here. Use the data to answer the following questions only. You do not have to analyze the data. Show proof when you make...
-
Split Corporation manufactures products X, Y, and Z from a joint production process. Joint costs are allocated to products based on relative sales values of the products at the split-off point....
-
A television show conducted an experiment to study what happens when buttered toast is dropped on the floor. When 44 buttered slices of toast were dropped, 28 of them landed with the buttered side up...
-
The mean daily rainfall between January 1, 2007, through January 1, 2009, at Pismo Beach, California, was 0.02 inches with a standard deviation of 0.11 inches. Based on this information, do you think...
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
Form the operator A 2 if A = x d/dx. Be sure to include an arbitrary function on which the operator acts.
-
Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? a. b. c. 1 cose sin e- sin e de...
-
Use a Fourier series expansion to express the function f (y) = y 2 , b ¤ y ¤ b, in the form Obtain d 0 and the first five pairs of coefficients c n and d n . cos f0) -d0+ , sin |+ dc...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...

Study smarter with the SolutionInn App


