You may need Table 7.2 to answer the following questions. a. Which is the stronger base, Cl
Question:
You may need Table 7.2 to answer the following questions.
a. Which is the stronger base, Cl2 or H2O?
b. Which is the stronger base, H2O or NO22?
c. Which is the stronger base, CN2 or OC6H5?
Table 7.2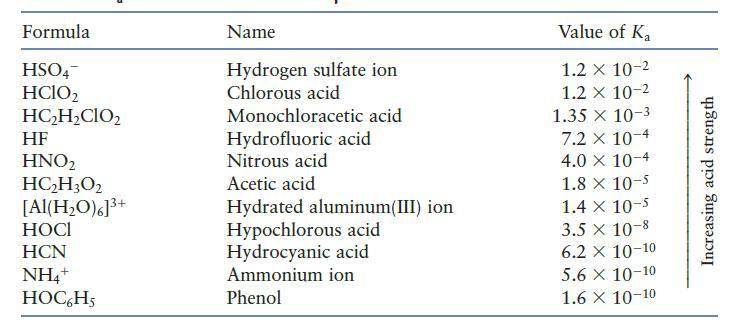
Transcribed Image Text:
Formula HSO4 HClO2 HC₂H₂CIO₂ HF HNO₂ HC₂H3O2 [Al(H₂O)6]³+ HOCI HCN NH4+ HOC6H5 Name Hydrogen sulfate ion Chlorous acid Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of Ka 1.2 x 10-2 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 X 10-4 1.8 x 10-5 1.4 x 10-5 3.5 x 10-8 6.2 X 10-10 5.6 X 10-10 1.6 X 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 27% (11 reviews)
a The pKa of Cl2 is 36 while the pKa of H2O is 1574 Since Cl2 has ...View the full answer

Answered By

Hande Dereli
Enthusiastic tutor, skilled in ACT and SAT tutoring. Raised one student's score on the SATs from 1100 combined to 1400. Graduated with a 3.9 GPA from Davidson College and led a popular peer tutoring group for three years. Scored in the top 0.06% in the nation on the SATs. The real reason I'm the one to help you nail the test? Results. Clients invariably praise my ability to listen and communicate in a low-stress, fun way. I think it's that great interaction that lets me raise retest SAT scores an average of 300 points.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Answer the following questions about internal control over cash payments: 1. Payment by check carries three controls over cash. What are they? 2. Suppose a purchasing agent receives the goods that he...
-
Answer the following questions based on Tables 5P-1 and 5P-2. a. What is the quantity demanded at $10? What is the quantity supplied at $10? b. What is the quantity demanded at $25? What is the...
-
Answer the following questions based on two assumptions: (1) Inflation increases the prices of all goods by 20%. (2) Ina's income increases from $50,000 to $55,000. a. Has Ina's budget line become...
-
How can staff review the effectiveness of their work, the services they provide and the social and cultural factors impacting on clients, groups or communities?
-
Suppose that X and Y are independent random variables. Suppose that X has a discrete distribution concentrated on finitely many distinct values with p.f. f1. Suppose that Y has a continuous...
-
The reaction of ethyl butanoate with sodium ethoxide in CH 3 CH 2 OH gives (a) (b) (c) (d) CH;CH,CH,CHCHCO,CH,CH3 H,CH3
-
Kimberly is divorced and the custodial parent of a three-year-old girl named Bailey. Kimberly and Bailey live with Kimberlys parents, who pay all the costs of maintaining the household (such as...
-
Sammys Pizza opened on January 1, 2016. Sammys reported the following for cash revenues and cash expenses for the years 2016 to 2018: Required a. What would Sammys Pizza report for net income and...
-
A family friend has asked your help in analyzing the operations of three anonymous companies operating in the same service sector industry. Supply the missing data in the table below: (Loss amounts...
-
The bank offered Annette a $380,000, 30-year mortgage at 3.54%. She is deciding whether to purchase 2 points to reduce her APR by 0.25% per point. Each point will cost 1% of the loan value. a....
-
Write balanced equations that describe the following reactions. a. The dissociation of perchloric acid in water. b. The dissociation of propanoic acid (CH 3 CH 2 CO 2 H) in water. c. The dissociation...
-
Consider the following statements. Write out an example reaction and K expression that are associated with each statement. a. The autoionization of water. b. An acid reacts with water to produce the...
-
1. Individual analysis: Read these sets of statements on your own, without discussing them with colleagues. In each case decide, as the supervisor: Which of the four statements is the best, and why?...
-
What are knowledge or innovation workers? What are the key elements of professional practice, work environment and work design needed to support the productivity and creativity of knowledge or...
-
What problem does this concept solve or what pain does it alleviate and how compelling is the problem? 2. Who is your specific target customer? 3. How do they currently meet this need for themselves...
-
Consider a person standing in a room where the average wall temperature is 20 C. This person is trying to reach the "thermal comfort" by adjusting the A/C air temperature. Find out the appropriate...
-
If WHO, the World Health Organization,defines health as a state of completephysical, mental and social well-being and not merely the absenceof disease and infirmity (WHO, 2011)and wellness is...
-
As a manager, you want to find a way to motivate Nate and increase his engagement and job satisfaction in the workplace. Drawing upon a behavioral theory of motivation, discuss how you, as a manager,...
-
If Earth didnt spin on its axis but still revolved around the Sun, would the Sun set on the eastern horizon, on the western horizon, or not at all?
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
What volume of 0.100 M Na 3 PO 4 is required to precipitate all of the lead(II) ions from 150.0 mL of 0.250 M Pb(NO 3 ) 2 ?
-
A 1.00-g sample of an alkaline earth metal chloride is treated with excess silver nitrate. All of the chloride is recovered as 1.38 g of silver chloride. Identify the metal.
-
A mixture contains only NaCl and Al 2 (SO 4 ) 3 . A 1.45- g sample of the mixture is dissolved in water, and an excess of NaOH is added, producing a precipitate of Al(OH) 3 . The precipitate is...
-
Nelo Partnership had three partners, whose capital balances on June 30 were as follows: Jack $50,000, Andy $35,000, Nick $22,000. The profit-sharing ratio is 6:4:2 (Jack, Andy, Nick). On July 1,...
-
Alex buys a Blu-ray disc costing $14.49. Use the table below to find the sales tax on this item. Amount of Sale ($) Tax ($) 13.70 13.89 0.69 13.90 14.09 0.70 14.10 14.29 0.71 14.30 14.49 0.72 14.50...
-
Show partial income statements through gross margin for all three methods, assuming both products are further processed into Current Attempt in Progress It's mind - boggling the number of products...

Study smarter with the SolutionInn App


