You may need Table 7.2 to answer the following questions. a. Which is the stronger acid, HCl
Question:
You may need Table 7.2 to answer the following questions.
a. Which is the stronger acid, HCl or H2O?
b. Which is the stronger acid, H2O or HNO2?
c. Which is the stronger acid, HCN or HOC6H5
Table 7.2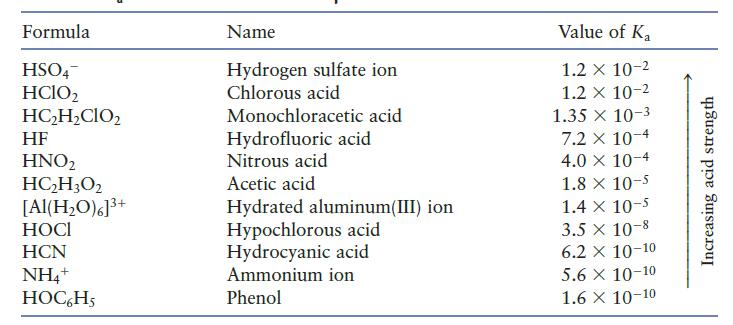
Transcribed Image Text:
Formula HSO4 HClO2 HC₂H₂CIO₂ HF HNO₂ HC₂H3O2 [Al(H₂O)6]³+ HOCI HCN NH4+ HOC6H5 Name Hydrogen sulfate ion Chlorous acid Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of Ka 1.2 x 10-2 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 × 10-4 1.8 x 10-5 1.4 x 10-5 3.5 x 10-8 6.2 X 10-10 5.6 X 10-10 1.6 X 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
aHCl has higher ka va...View the full answer

Answered By

Godswill Okorie
M.Sc chemistry specialization in organic chemistry, B.ed .I am having industry experience of seven years working with Ranbaxy nd Shimadzu analytical India by working as an application chemistry.I am having good practical experience on chromatography techniques,which later helped me in my teaching.I worked as PGT chemistry teacher with KV and APS.
As a teacher I was able to achieve good results with my students.I used to take 11th and 12th chemistry and science to classes 7th ,8th and ninth. While teaching I used to guide students for various carrier opportunities.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which is the stronger acid in each of the following pairs? Explain your reasoning. (a) Phenol or p-hydroxybenzaldehyde (b) m-Cyanophenol or p-cyanophenol (c) o-Fluorophenol or p-fluorophenol
-
Which is the stronger acid in each of the following pairs?
-
Answer the following questions based on Tables 5P-1 and 5P-2. a. What is the quantity demanded at $10? What is the quantity supplied at $10? b. What is the quantity demanded at $25? What is the...
-
The need to be liked and to stay on good terms with most other people is the need for? a. Affiliation b. Power c. None of the above d. Achievement
-
Suppose that, on a particular day, two persons A and B arrive at a certain store independently of each other. Suppose that A remains in the store for 15 minutes and B remains in the store for 10...
-
Is it possible for two different diastereomeric aldoses to give the same product upon KilianiFischer chain elongation? Why or why not?
-
Assume he has no itemized deductions. b. Which engagement maximizes Camerons after-tax cash flow? Explain.
-
Keppel Corporation manufactures and sells three different models of exterior doors. Although the doors vary in terms of quality and features, all are good sellers. Keppel is currently operating at...
-
Required information Trey Monson starts a merchandising business on December 1 and enters into three inventory purchases: Purchases on December 7 Purchases on December 14 Purchases on December 21 10...
-
The client, Mrs. Tatum, purchased a new microwave oven from Inki Appliances Company. No written or oral warranty was given when the sale was made. The microwave stopped working one week after Mrs....
-
The salt BX, when dissolved in water, produces an acidic solution. Which of the following could be true? (There may be more than one correct answer.) a. The acid HX is a weak acid. b. The acid HX is...
-
Match the following pH values: 1, 2, 5, 6, 6.5, 8, 11, 11 and 13 with the following chemicals (of equal concentration): HBr, NaOH, NaF, NaCN, NH 4 F, CH 3 NH 3 F, HF, HCN, and NH 3 . Answer this...
-
56. In 2019, Sven is single and has $120,000 of regular taxable income. He itemizes his deductions as follows: real property tax of $2,000, state income tax of $4,000, and mortgage interest expense...
-
Toro Corp. reports the following two years of balance sheets and some additional information. 2019 2018 Cash S 92,915 $ 31,355 Accounts receivable 94,000 80,850 Inventory 179,000 157,600 Prepaid...
-
The Westchester Chamber of Commerce periodically sponsors public service seminars and programs. Currently, promotional plans are under way for this year's program. Advertising alternatives include...
-
Mastery Problem: Differential Analysis and Product Pricing WoolCorp WoolCorp buys sheep's wool from farmers. The company began operations in January of this year, and is making decisions on product...
-
Ross Co. is an oil and gas company located in the Western United States. Ross follows U.S. GAAP in recording and reporting its financial transactions and has a year-end of 12/31. During the fiscal...
-
The following unadjusted trial balance is for ACE CONSTRUCTION CO. as of the end of its 2017 fiscal year. The June 30, 2016, credit balance of the owners capital account was $57,000, and the owner...
-
Rank in order of increasing average distance from the Sun: (a) Kuiper belt objects, (b) Asteroids, (c) Oort cloud objects.
-
Research corporate acquisitions using Web resources and then answer the following questions: Why do firms purchase other corporations? Do firms pay too much for the acquired corporation? Why do so...
-
A compressed-gas cylinder contains 1.00 10 3 g of argon gas. The pressure inside the cylinder is 2050. psi (pounds per square inch) at a temperature of 18 o C. How much gas remains in the cylinder...
-
Equal moles of sulfur dioxide gas and oxygen gas are mixed in a flexible reaction vessel and then sparked to initiate the formation of gaseous sulfur trioxide. Assuming that the reaction goes to...
-
Silane (SiH 4 ) is the silicon analogue of methane (CH 4 ). It is prepared industrially according to the following equations: Si(s) + 3HCl(g) HSiCl 3 (l) + H 2 (g) 4HSiCl 3 (l) SiH 4 (g) + 3SiCl 4...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...

Study smarter with the SolutionInn App


