Question:
You want to check the accuracy of a pH meter used for titrations and so calculate the expected pH at various points in a titration. The analyte consists of 25.00 mL of 0.250 m NaOH(aq), and the titrant is 0.340 m HCl(aq). Calculate
(a) The pH of the original analyte solution and
(b) The pH after the addition of 5.00 mL of the acid titrant.
ANTICIPATE You should expect the pH to decrease from its initial value as acid is added.
PLAN For part (a), determine the pOH of the solution and convert it to the pH. For part (b), follow the procedure outlined in Toolbox 6H.1.
What should you assume? Assume that so much acid or base is present that the autoprotolysis of water does not contribute significantly to the pH.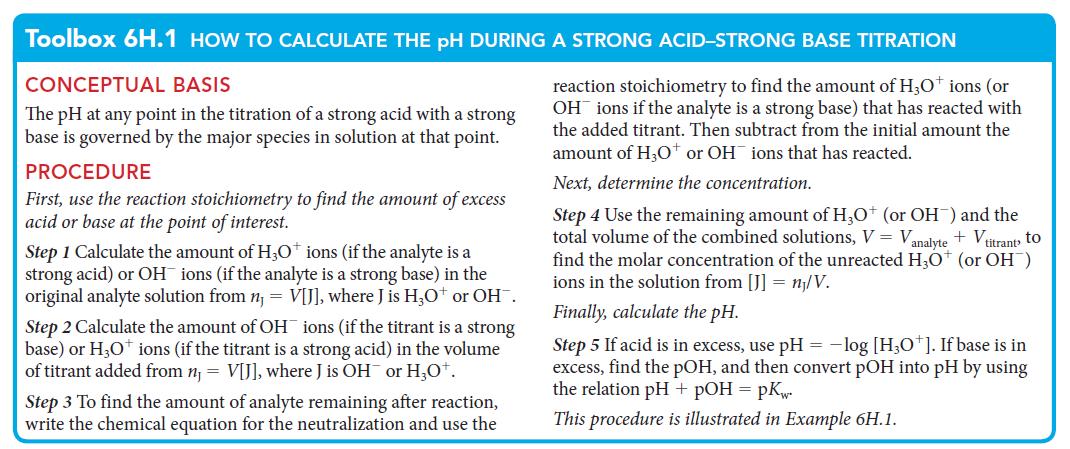
Transcribed Image Text:
Toolbox 6H.1 HOW TO CALCULATE THE PH DURING A STRONG ACID-STRONG BASE TITRATION
CONCEPTUAL BASIS
The pH at any point in the titration of a strong acid with a strong
base is governed by the major species in solution at that point.
PROCEDURE
reaction stoichiometry to find the amount of H3O+ ions (or
OH ions if the analyte is a strong base) that has reacted with
the added titrant. Then subtract from the initial amount the
amount of H₂O+ or OH ions that has reacted.
Next, determine the concentration.
First, use the reaction stoichiometry to find the amount of excess
acid or base at the point of interest.
Step 1 Calculate the amount of H3O+ ions (if the analyte is a
strong acid) or OH ions (if the analyte is a strong base) in the
original analyte solution from n, V[J], where J is H3O+ or OH™.
Step 2 Calculate the amount of OH ions (if the titrant is a strong
base) or H3O+ ions (if the titrant is a strong acid) in the volume
of titrant added from n₁ = V[J], where J is OH or H₂O+.
Step 3 To find the amount of analyte remaining after reaction,
write the chemical equation for the neutralization and use the
Step 4 Use the remaining amount of H3O+ (or OH¯) and the
total volume of the combined solutions, V = Vanalyte + Vitrant, to
find the molar concentration of the unreacted H3O+ (or OH)
ions in the solution from [J] = n/V.
Finally, calculate the pH.
Step 5 If acid is in excess, use pH = -log [H3O+]. If base is in
excess, find the pOH, and then convert pOH into pH by using
the relation pH + pOH = pKw.
This procedure is illustrated in Example 6H.1.







