As a part of your research program on formic acid, you need to titrate a solution of
Question:
As a part of your research program on formic acid, you need to titrate a solution of formic acid with sodium hydroxide solution and want to know what to expect. Calculate the pH of
(a) 0.100 m HCOOH(aq) and
(b) The solution resulting when 5.00 mL of 0.150 m NaOH(aq) is added to 25.00 mL of the acid. Use Ka = 1.8 * 10–4 for HCOOH.
ANTICIPATE When the strong base is added, some of the weak acid is neutralized, so you should expect the pH to rise from part (a) to part (b).
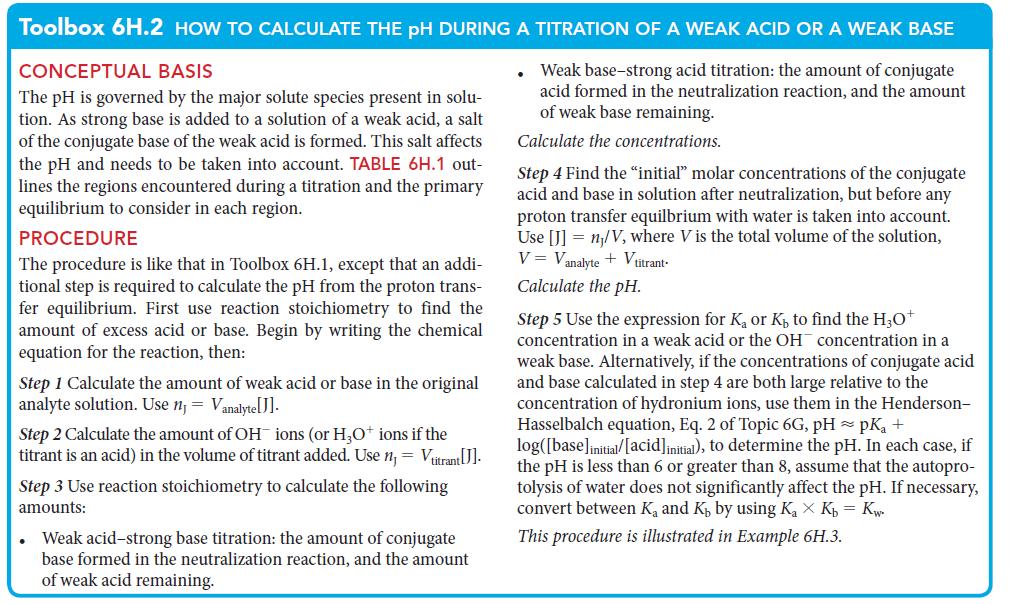
PLAN For part (a), use the procedure in Toolbox 6D.1. For part (b), find the pH using the procedure in Toolbox 6H.2.
What should you assume? Assume that the autoprotolysis of water does not contribute significantly to the pH and that the weak acid formic acid undergoes only a small degree of deprotonation.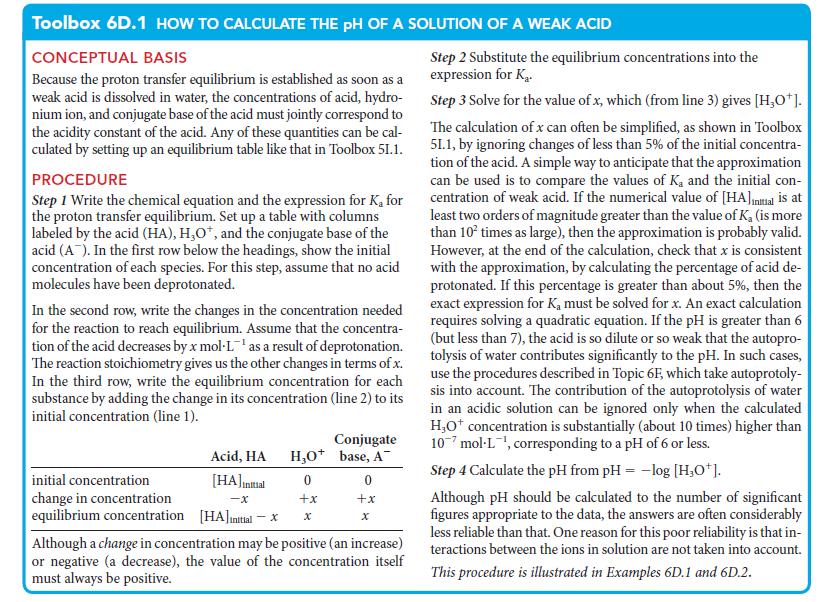
Toolbox 6D.1 HOW TO CALCULATE THE pH OF A SOLUTION OF A WEAK ACID CONCEPTUAL BASIS Because the proton transfer equilibrium is established as soon as a weak acid is dissolved in water, the concentrations of acid, hydro- nium ion, and conjugate base of the acid must jointly correspond to the acidity constant of the acid. Any of these quantities can be cal- culated by setting up an equilibrium table like that in Toolbox 51.1. PROCEDURE Step 1 Write the chemical equation and the expression for K₂ for the proton transfer equilibrium. Set up a table with columns labeled by the acid (HA), H₂O*, and the conjugate base of the acid (A). In the first row below the headings, show the initial concentration of each species. For this step, assume that no acid molecules have been deprotonated. In the second row, write the changes in the concentration needed for the reaction to reach equilibrium. Assume that the concentra- tion of the acid decreases by x mol-L¹ as a result of deprotonation. The reaction stoichiometry gives us the other changes in terms of x. In the third row, write the equilibrium concentration for each substance by adding the change in its concentration (line 2) to its initial concentration (line 1). Acid, HA H₂O 0 +x x [HA]Initial -X [HA] initial - X Conjugate base, A initial concentration change in concentration equilibrium concentration Although a change in concentration may be positive (an increase) or negative (a decrease), the value of the concentration itself must always be positive. 0 +x Step 2 Substitute the equilibrium concentrations into the expression for Ka Step 3 Solve for the value of x, which (from line 3) gives [H₂O*]. The calculation of x can often be simplified, as shown in Toolbox 51.1, by ignoring changes of less than 5% of the initial concentra- tion of the acid. A simple way to anticipate that the approximation can be used is to compare the values of Ka and the initial con- centration of weak acid. If the numerical value of [HA]inmal is at least two orders of magnitude greater than the value of K₂ (is more than 10² times as large), then the approximation is probably valid. However, at the end of the calculation, check that x is consistent with the approximation, by calculating the percentage of acid de- protonated. If this percentage is greater than about 5%, then the exact expression for K, must be solved for x. An exact calculation requires solving a quadratic equation. If the pH is greater than 6 (but less than 7), the acid is so dilute or so weak that the autopro- tolysis of water contributes significantly to the pH. In such cases, use the procedures described in Topic 6F, which take autoprotoly- sis into account. The contribution of the autoprotolysis of water in an acidic solution can be ignored only when the calculated H₂O* concentration is substantially (about 10 times) higher than 107 mol-L¹, corresponding to a pH of 6 or less. Step 4 Calculate the pH from pH = -log [H₂O*]. Although pH should be calculated to the number of significant figures appropriate to the data, the answers are often considerably less reliable than that. One reason for this poor reliability is that in- teractions between the ions in solution are not taken into account. This procedure is illustrated in Examples 6D.1 and 6D.2.
Step by Step Answer:
a The proton transfer equilibrium is HCOOHaq HOl HOaq HCOaq From H3O KHA initial 2 and pH log H0 pH log18 X 104 x 01002 237 b The neutralization react...View the full answer



Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Students also viewed these Sciences questions
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Although there are extensive tables available for the pK a of weak acids, you might be dealing with an unknown acid or a known acid at an unlisted temperature. You could then use a procedure like...
-
The yields of nine batches of a chemical process were measured and a sample mean of 2.843 and a sample standard deviation of 0.150 were obtained. The experimenter presented a confidence interval of...
-
Closing down divisions. Aristide corporation has four operating divisions. During the first quarter of 2009, the company reported total income from operations of $61,000 and the following results for...
-
Show that in choosing the so-called best subset model from a series of candidate models, if the model is chosen that has the smallest s2, this is equivalent to choosing the model with the smallest...
-
Suppose your college or organization is considering a new project to develop an information system that would allow all employees, students, and customers to access and maintain their own human...
-
Bigelow Corporation has total assets of $850,000, sales of $1,350,000, and one class of common stock with 375 shareholders, and a class of preferred stock with 250 shareholders, both of which are...
-
Imprudential, Inc., has an unfunded pension liability of $574 million that must be paid in 15 years. To assess the value of the firms stock, financial analysts want to discount this liability back to...
-
Using the SAS data set vitals, create two temporary SAS data sets called Subset_A and Subset_B. Include in both of these data sets a variable called Combined equal to .001 times WBC plus RBC....
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Muscles produce lactic acid during exercise. Calculate the pH, pOH, and percentage deprotonation of the following aqueous solutions of lactic acid, CH 3 CH(OH)COOH: (a) 0.11 m; (b) 3.7 * 10 3 m; (c)...
-
Repeat Problem 6 assuming the corporation is an S corporation.
-
Consider a two-period model in which an individual needs to decide how much to consume in the present, c, and how much to consume in the future, c. The individual receives income, y, in the present,...
-
Koopman Company began operations on January 1, 2015, and uses the FIFO inventory method for financial reporting and the average cost inventory method for income taxes. At the beginning of 2017,...
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
1.Basis of Islamic Accounting Theory The history of the birth of Islamic accounting is inseparable from the development of Islam, the obligation to record non-cash transactions (see QS. Al-Baqarah:...
-
What kind of information can you provide from the label on the door frame of a car? A scene of a car from drivers side a. factory recommended mileage a year b. factory recommended tire pressure c....
-
The following normal quantile plot was created from the times that it took 166 bicycle riders to complete the stage 11 time trial, from Grenoble to Chamrousse, France, in the 2001 Tour de France...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
Show that the set of functions n () = e in , 0 2is orthogonal if n is an integer. To do so, you need to 2 0 * m () n () = d = 0 for m n if n and m are intergers.
-
In normalizing wave functions, the integration is over all space in which the wave function is defined. a. Normalize the wave function x (a x) y (b y) over the range 0 x a, 0 y b. The element...
-
Operate with a. /x + /y + /z b. 2 /x 2 + 2 /y 2 + 2 /3 2 on the function Ae ik1x e ik2y e ik3z . Is the function an eigenfunction of either operator? If so, what is the eigenvalue?
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...

Study smarter with the SolutionInn App


