The adiabatic exothermic irreversible gas-phase reaction 2A + B 2C is to be carried out in
Question:
The adiabatic exothermic irreversible gas-phase reaction 2A + B → 2C is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in Figure P2-7B.
Figure P2-7B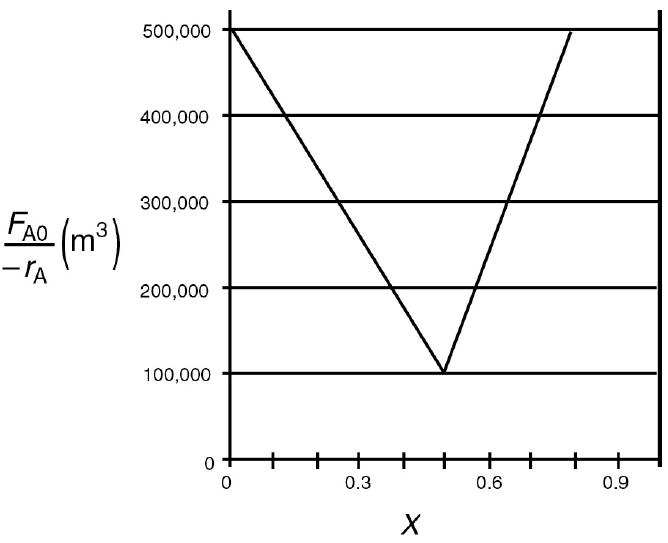
A graph plots the values of volume for different conversion values. The horizontal axis represents the conversion in the range 0 to 0.9 in increments of 0.1. The vertical axis represents the volume with molar flow rate as F subscript A0, ranging from 0 to 500,000 in increments of 10. The rate decreases steadily in the beginning and from 0.5 of X it increases. The maximum volume observed is 500,000 cubic meters and minimum is 100,000 cubic meters.
a. What PFR volume is necessary to achieve 50% conversion?
b. What CSTR volume is necessary to achieve 50% conversion?
c. What is the volume of a second CSTR added in series to the first CSTR (Part b) necessary to achieve an overall conversion of 80%?
d. What PFR volume must be added to the first CSTR (Part b) to raise the conversion to 80%?
e. What conversion can be achieved in a 6 × 104 m3 CSTR? In a 6 × 104 m3 PFR?
f. Think critically (cf. Introduction, Section H, page xxix) to critique the answers (numbers) to this problem.
Step by Step Answer:






