The following reactions are taking place in a 2000-dm 3 liquid-phase batch reactor under a pressure of
Question:
The following reactions are taking place in a 2000-dm3 liquid-phase batch reactor under a pressure of 400 psig:![]()
The initial temperature is 450 K and the initial concentrations of A, B, and C are 1.0, 0.5, and 0.2 mol/dm3, respectively. The coolant flow rate was at its maximum value so that Ta1 = Ta2 = Ta = 400 K, so that the product, the exchange area, and the overall heat transfer coefficient, UA, is UA = 100 cal/s · K.
(a) If Qr > Qg at time t = 0, and there is no failure of the heat exchange system, is there any possibility that the reactor will run away? Explain.
(b) What is Qr at t = 0?
(c) What is Qg at t = 0?
(d) What is the initial rate of increase in temperature, (i.e., dT/dt) at t = 0?
dTdt=__________[Numericla Answer]
(e) Suppose that the ambient temperature Ta is lowered from 400 K to 350 K; what is the initial rate of reactor temperature change?
dTdt=__________[Numerical Answer]
Plot the temperatures and all the concentrations as a function of time up to t = 1000 seconds.
(f) A suggestion was made to add 50 moles of inerts at a temperature of 450 K. Will the addition of the inerts make runaway more likely or less likely? How? Show
quantitatively.
Additional information:
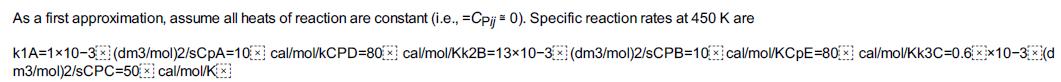
Step by Step Answer:






