A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration
Question:
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here.
Determine the molar mass and pKa of the acid.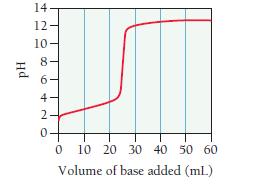
Transcribed Image Text:
Hd 14 12- 10 8 6- Na 00 4- 0 0 10 20 30 40 50 60 Volume of base added (ml)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
pK...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration curve is shown here. Determine the molar mass and pKa of the acid. Hd 14 12- 10- 06+NO 8- 4- 2 20...
-
Find the analytic tan te=4+Pv. 37 co20-9 Coso +2.
-
A 0.1276-g sample of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0633 M NaOH solution. The volume of base required to bring the solution to the equivalence point...
-
The following question is designed to highlight key concepts from the Loyalty Programs topic article titled, "StarBUCKS, Loyalty, and Breakage" (Nevraumont 2019). Q. Author's position. "If you hire...
-
Peters Company produces golf discs which it normally sells to retailers for $7 each. The cost of manufacturing 20,000 golf discs is: Materials ........ $ 10,000 Labor ......... 30,000 Variable...
-
Why cant we use random assignment in the study of homophobia, and what effect will that have on the conclusions we are allowed to draw?
-
P 22-5 Statement of activitiesNongovernmental not-for-profit organization The following information was taken from the accounts and records of the Community Society, a nongovernmental not-for-profit...
-
Layla meets with her insurance agent, Trong, to discuss her insurance needs. Layla has an after-tax income of $3,800 per month, her rent is $1,200, and her other expenses total $1,500 per month....
-
1) On February 7, 2021, Las Marias Shop purchased several inventory units at $30.00 each. The initial selling price was $40.00. The following table shows the changes in the sales price of these units...
-
A 20.0-mL sample of 0.115 M sulfurous acid (H 2 SO 3 ) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Consider a 30-year, $145,000 mortgage with a 6.1 percent interest rate. After eight years, the borrower (the mortgage issuer) pays it off. How much will the lender receive?
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Mesa Cheese Company has developed a new cheese slicer called Slim Slicer. The company plans to sell this slicer through its catalog, which it issues monthly. Given market research, Mesa believes that...
-
In the synthesis of the keto acid just given, the dicarboxylic acid decarboxylates in a specific way; it gives Explain. HO rather than HO
-
A point source S is a perpendicular distance R away from the center of a circular hole of radius a in an opaque screen. If the distance from S to the periphery of the hole is (R + ), show that...
-
Starting with Eq. (9.53) for the transmitted wave, compute the flux density, that is, Eq. (9.54). , = Egelo tt' Eoeiot 1 pPe-i (9.53) 14(t')? I; (1 + r4) 2r (9.54) cos 8
-
A form of the Jamin Interferometer is illustrated in Fig. P.9.52. How does it work? To what use might it be put? Figure P.9.52
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


