A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration
Question:
A 0.446-g sample of an unknown monoprotic acid is titrated with 0.105 M KOH. The resulting titration curve is shown here.
Determine the molar mass and pKa of the acid.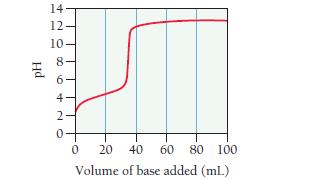
Transcribed Image Text:
Hd 14 12- 10- ∞06+NO 8- 4- 2 20 40 60 80 100 Volume of base added (mL) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the molar mass of the acid 1 Calculate the moles of KOH used in the titration 2 Since t...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pK a of the acid. Hd 14 12- 10 8 6- Na 00 4- 0...
-
Find the analytic tan te=4+Pv. 37 co20-9 Coso +2.
-
A 0.5224-g sample of an unknown monoprotic acid was titrated with 0.0998 M NaOH. The equivalence point of the titration occurred at 23.82 mL. Determine the molar mass of the unknown acid.
-
Why " Kodak " is unsuccessful in implementing a strategy. Can you prepare a critical examination of the strategy to address the following questions about Kodak. What was the strategy and why do you...
-
Winters Inc. has been manufacturing its own shades for its table lamps. The company is currently operating at 100% of capacity. Variable manufacturing overhead is charged to production at the rate of...
-
Demonstrate that, because we have equal sample sizes, I would have arrived at the same answer in Section 14.10 if I had not pooled the variances, although the degrees of freedom would probably differ.
-
P 22-4 Journal entries and statement of activitiesNongovernmental not-for-profit college The following information relates to revenues and expenses for a private not-for-profit college: Tuition and...
-
The franchise arrangement between McDonalds and its franchisees is summarized in the following note from McDonalds 2007 annual report. Individual franchise arrangements generally include a lease and...
-
Current Attempt in Progress Skysong, Inc. completed the following merchandising transactions in the month of May. At the beginning of May, the ledger of Skysong, Inc. showed Cash of $8,700 and Common...
-
A 20.0-mL sample of 0.115 M sulfurous acid (H 2 SO 3 ) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
As stated in Chapter 23, mammalian cells can become resistant to the lethal action of methotrexate by the selective survival of cells containing increases in dihydrofolate reductase gene copy number...
-
Q.9 Prepare a cash flow statement using the indirect method based on the following information: - Net Income: $150,000 - Depreciation Expense: $20,000 - Increase in Accounts Receivable: $10,000...
-
3.11 (a) Find the order of the elements 2, 7, 10 and 12 in F17. (b) Find the order of the elements a, a, a + 1 and a3 + 1 in F16, where a is a root of 1+x+x4.
-
You have been recently hired to lead a Project to relocate your main Distribution Centre (DC) from Calgary, Alberta to St. John's, Newfoundland. As the Project Manager, try to complete a project plan...
-
Males Mean: 69.6 Standard Deviation: 11.3 For males, find P90, which is the pulse rate separating the bottom 90% from the top 10%.
-
Statistics Assignments Using Excel Assignment #4: Measures of Variability Part I Below are ACT composite scores from 20 randomly selected college students. 15 33 20 25 21 24 17 16 20 25 26 21 21 17...
-
Chen Company's Small Motor Division manufactures a number of small motors used in household and office appliances. The Household Division of Chen then assembles and packages such items as blenders...
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a seven-year operating lease agreement. The contract calls for quarterly rent payments of $25,000 each....
-
A glass camera lens with an index of 1.55 is to be coated with a cryolite film (n 1.30) to decrease the reflection of normally incident green light ( 0 = 500 nm). What thickness should be deposited...
-
Using Fig. 9.73, which depicts the geometry of the Shuttle radar interferometer, show that z(x) = h - r 1 cos θ Then use the Law of Cosines to establish that Eq. (9.108) is correct. Fig....
-
Given that the mirrors of a FabryPerot Interferometer have an amplitude reflection coefficient of r = 0.894 4, find (a) The coefficient of finesse, (b) The half-width, (c) The finesse, and, (d) The...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


