A nitrogen gas laser pulse with a wavelength of 337 nm contains 3.83 mJ of energy. How
Question:
A nitrogen gas laser pulse with a wavelength of 337 nm contains 3.83 mJ of energy. How many photons does it contain?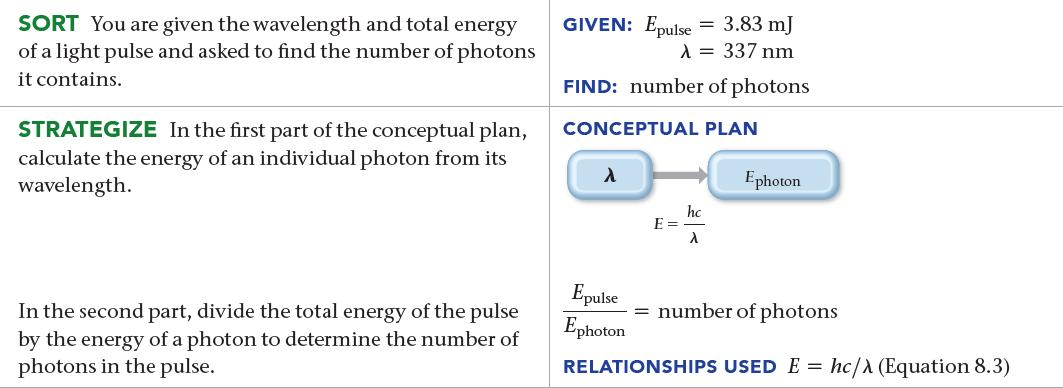
Transcribed Image Text:
SORT You are given the wavelength and total energy of a light pulse and asked to find the number of photons it contains. STRATEGIZE In the first part of the conceptual plan, calculate the energy of an individual photon from its wavelength. In the second part, divide the total energy of the pulse by the energy of a photon to determine the number of photons in the pulse. GIVEN: Epulse = 3.83 mJ λ = 337 nm FIND: number of photons CONCEPTUAL PLAN λ E = hc λ Ephoton Epulse Ephoton = number of photons RELATIONSHIPS USED E= hc/> (Equation 8.3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
337 nm X Ephoton hc 10 m 1 nm 337 x 107 m 6626 X 1034Js 3...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 1-second pulse of a red laser pointer with a wavelength of 635 nm contains 5.0 mJ of energy. How many photons does it contain?
-
A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is? (a) 0.10 W, (b) LOW?
-
A laser used to weld detached retinas puts out 28-ms-long pulses of 640-nm light which average 0.68-W output during a pulse. How much energy can be deposited per pulse and how many photons does each...
-
(1) Choose all of the following statements that are correct about the time evolution of a general wave function: (I) The time evolution of a general wave function is governed by the Hamiltonian...
-
In its 2006 annual report, McDonalds Corporation reports beginning total assets of $30.0 billion; ending total assets of $29.0 billion; net sales of $21.6 billion, and net income of $3.5 billion. (a)...
-
Which of the following statements gives the best description of nowcasting? A. This method is used to forecast future trends in economic variables based on their past and current values. B. This...
-
How do risk and inflation impact interest rates in the economy? AppendixLO1
-
The comparative balance sheet of Mavenir Technologies Inc. for December 31, 2010 and 2009, is shown as follows: The following additional information was taken from the records: a. The investments...
-
Financial Reporting Problem The Procter & Gamble Company (P&G) The financial statements of P&G are presented in Appendix B. The company's complete annual report, including the notes to the financial...
-
Kansas Corp., an American company, has a payment of 5 million due to Tuscany Corp. one year from today. At the prevailing spot rate of 0.90 /$, this would cost Kansas $5,555,556, but Kansas faces the...
-
Which wavelength of light has the highest frequency? a) 10 nm b) 10 mm c) 1 nm d) 1 mm
-
Which statement best describes the differences between a bright green laser and a dim red laser? (a) The two lasers emit light of the same frequency, and the light from the green laser has a greater...
-
Why the different requirement for negotiation?
-
4. (15pt) A group of students were asked if they have ever driven after drinking. They also were asked, "How many days per month do you drink at least two beers?" In the following discussion, 7 = the...
-
discuss how might you apply the concepts of Total Quality (TQ) to your personal and work environment. Consider your relations with others and your daily activities interactions with. Share the...
-
Dr. Bernstein wants to expand his radiology practice. Dr. Bernstein is researching various local banks for the best certificate of deposit rate to fund his expansion. One bank is willing to offer him...
-
An airplane is flying with a velocity of 240 m/s at an angle of 30.0 with the horizontal, as the drawing shows. When the altitude of the plane is 2.4 km, a flare is released from the plane. The flare...
-
Katsura Corporation incurred pre - operating costs: Investigatory expenses of $ 1 8 , 0 0 0 New employee training $ 2 5 , 0 0 0 Advertising $ 1 0 , 0 0 0 Land and building for use as a retail store...
-
The time to deliver packaged containers by a logistics company is found from samples of size 4. The mean and standard deviation of delivery times is estimated to be 140 hours and 6 hours,...
-
Using (1) or (2), find L(f) if f(t) if equals: t cos 4t
-
Show that two of the set of four equivalent orbitals appropriate for sp 3 hybridization, And are orthogonal. W. =-62, + 2p, + 2, + 2p,) (: - O2p. 2p, + 2p,)
-
Show that the water hybrid bonding orbitals given by a = 0.55 2Pz + 0.71 2 px -0.45 2 ps b = 0.55 2pz - 0.71 2px - 0.45 2s are orthogonal.
-
Predict which of the bent molecules, BH 2 or NH 2 , should have the larger bond angle on the basis of the Walsh correlation diagram in Figure 24.11. Explain your answer. Figure 24.11 16, 1Tu + 2a,...
-
On October 1, 2020 Macklin Corporation issued 5%, 10-year bonds with a face value of $6,000,000 at 104. Interest is paid on October 1 and April 1, with any premiums or discounts amortized on a...
-
You have been hired by Internal Business Machines Corporation (IBM) in their capital budgeting division. Your first assignment is to determine the free cash flows and NPV of a proposed new type of...
-
Suppose that the dollar-mark 6 months forward rate is $1.275/Mark. Suppose that the dollar-mark forward premium is 5%. Calculate the spot rate, $1=Mark_______ work to 4 decimal places.

Study smarter with the SolutionInn App


