Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq)
Question:
Balance each redox reaction occurring in acidic aqueous solution.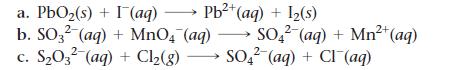
Transcribed Image Text:
a. PbO₂(s) + I (aq) →→→ Pb²+ (aq) + 1₂(s) 2+ b. SO32 (aq) + MnO4 (aq) → SO42² (aq) + Mn²+ (aq) 2- c. S₂03² (aq) + Cl₂(g) → SO4² (aq) + Cl¯ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a PbO s 21 aq 4H aq 2 Pb aq I2...View the full answer

Answered By

PU Student
cost accounting
financial accounting
auditing
internal control
business analyst
tax
i have 3 years experience in field of management & auditing in different multinational firms. i also have 16 months experience as an accountant in different international firms. secondary school certification.
higher secondary school certification.
bachelors in mathematics.
cost & management accountant
4.80+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4 (aq) + Cl(aq) c. NO3(aq) + Sn+ (aq) 1(s) + NO(g) CIO3(aq) + Cl(g) Sn+ (aq) + NO(g)
-
Balance each redox reaction occurring in acidic aqueous solution. a. Zn(s) + Sn+ (aq) b. Mg(s) + Cr+ (aq) Zn+ (aq) + Sn(s) Mg+ (aq) + Cr(s) c. MnO4 (aq) + Al(s) Mn+ (aq) + A1+ (aq)
-
What is the type of the expressions computed on these two lines? 4 > 5 print (4>5)
-
Lightning Industries specializes in making Flash, a high-moisture, low-alkaline wax used to protect and preserve skis. The company began producing a new, improved brand of Flash on January 1....
-
Let X (t) be a modified version of the random telegraph process. The process switches between the two states X (t) = 1 and X (t) = 1 with the time between switches following exponential...
-
17. XYZ Corp. compensates executives with 10-year European call options, granted at the money. If there is a significant drop in the share price, the companys board will reset the strike price of the...
-
1. Why install an ERP? 2. Why not install an ERP? 3. Do you try to cost-benefit justify such a system, and, if so, how? 4. Are there corporate culture issues involved? 5. What degree of top...
-
Ace Hardware Corporation had the following classes of stock in Stockholders' Equity Preferred Stock, $100 par, $6%, non-cumulative stock, 5,000 shares issued Common Stock, $10 par, 50,000 shares...
-
Balance each redox reaction occurring in basic aqueous solution. a. HO(aq) + ClO(aq) CIO (aq) + O(g) b. Al(s) + MnO4 (aq) MnO(s) + Al(OH)4 (aq) c. Cl(g) Cl(aq) + CIO (aq)
-
How can the corrosion of iron be prevented?
-
Explain the terms PV and FV?
-
What is the formula for Bouley's coefficient of skewness?
-
What is the relation between orthocentre,circumcentre and centroid of a triangle?
-
When do we use Fourier transforms and Laplace transforms in RC/RL/RLC circuit analysis?
-
What are the protocols used in a drone?
-
How do we design a drone?
-
Homestead Oil Corp. was incorporated on January 1, 2016, and issued the following stock for cash: 800.000 shares of no-par common stock were authorized: 150.000 shares were issued on January 1, 2016,...
-
Suppose Green Network Energy needs to raise money to finance its new manufacturing facility, but their CFO does not think the company is financially capable of making the periodic interest payments...
-
Figure Q10.16 shows a popular demonstration involving a moving fluid. Here an air jet is aimed upward and levitates a small object such as a table-tennis ball. The ball is drawn to the center of the...
-
The recommended pressure in your cars tires is usually specified as a gauge pressure. If a tire has a gauge pressure of 15 lb/in. 2 , what is the absolute pressure in pascals?
-
Explain how there can be dust on a moving fan blade.
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


