Balance each redox reaction occurring in basic aqueous solution. a. HO(aq) + ClO(aq) CIO (aq) + O(g)
Question:
Balance each redox reaction occurring in basic aqueous solution.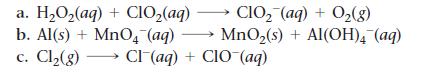
Transcribed Image Text:
a. H₂O₂(aq) + ClO₂(aq) CIO₂ (aq) + O₂(g) b. Al(s) + MnO4 (aq) → MnO₂(s) + Al(OH)4 (aq) c. Cl₂(g) →→→ Cl(aq) + CIO (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a HOaq 2 C1Oaq 2 OHaq ...View the full answer

Answered By

Sourindra Samanta
I'm teaching students as private tutor from last 2 years
Teaching math & science & advance biology in higher level
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O(g) Ag(CN) (aq) c. NO (aq) + Al(s) - NH3(g) + AlO (aq)
-
Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4 (aq) + Cl(aq) c. NO3(aq) + Sn+ (aq) 1(s) + NO(g) CIO3(aq) + Cl(g) Sn+ (aq) + NO(g)
-
Gabriele Enterprises has bonds on the market making annual payments, with seven years to maturity, a par value of $1,000, and selling for $974. At this price, the bonds yield 7.2 percent. What must...
-
Suppose you are a management consultant and a client asks you why companies include standard costs in their cost accounting systems. Prepare your response, listing several purposes for using standard...
-
Prove that convergence almost everywhere implies convergence
-
15. Consider Panels B and D in Figure 4. Using the information in each panel, compute the share price at each node for each bond issue.
-
Chain AB supports a horizontal, uniform steel beam having a mass per unit length of 85 kg/m. If the maximum tension in the cable is not to exceed 8kN, determine (a) The horizontal distance a from A...
-
In the periodic inventory system, the journal entry to record a purchase of merchandise on account is: Debit Merchandise Inventory Credit Cash Debit Merchandise Inventory Credit Account Payable Debit...
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq) + 1(s) 2+ b. SO32 (aq) + MnO4 (aq) SO42 (aq) + Mn+ (aq) 2- c. S03 (aq) + Cl(g) SO4 (aq) + Cl (aq)
-
What are the major differences between insertion sequences and transposons?
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
On May 4, 2016, Docker. Inc.. purchased 1,200 shares of its own common stock in the market at a price of $19.25 per share. On September 19, 2016, 700 of these shares were sold in the open market at a...
-
Refer to the data for problem 13-36 regarding Long Beach Pharmaceutical Company. Required: Compute each division's residual income for the year under each of the following assumptions about the...
-
A suction cup works by virtue of a vacuum that is created within the cup. When the cup is pressed against a flat surface, most of the air is forced out, leaving a region of very low pressure. If a...
-
Two blocks with the same volume but different densities are in a lake as shown in Figure Q10.14. Block m 1 floats at the surface with a portion above the water level, and block m 2 floats underwater....
-
The four tires on the authors car are inflated to an absolute pressure of 2.5 10 5 Pa. If the car has a mass of 1800 kg, what is the contact area between each tire and the ground?
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


