Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s)
Question:
Balance each redox reaction occurring in basic aqueous solution.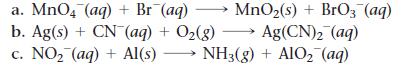
Transcribed Image Text:
a. MnO4 (aq) + Br (aq) → MnO₂(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O₂(g) → Ag(CN)₂ (aq) c. NO₂ (aq) + Al(s) - NH3(g) + AlO₂ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
the balanced redox reactions occurring in basic aqueous solution a 1 Assign oxidation numbers Mn 7 in MnO4 4 in MnO2 Br 1 in Br 5 in BrO3 2 Identify t...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in basic aqueous solution. a. HO(aq) + ClO(aq) CIO (aq) + O(g) b. Al(s) + MnO4 (aq) MnO(s) + Al(OH)4 (aq) c. Cl(g) Cl(aq) + CIO (aq)
-
Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4 (aq) + Cl(aq) c. NO3(aq) + Sn+ (aq) 1(s) + NO(g) CIO3(aq) + Cl(g) Sn+ (aq) + NO(g)
-
Given that log (2) 0.91 and log (5) 2.1, evaluate each of the following. Hint: use the properties of logarithms to rewrite the given logarithm in terms of the the logarithms of 2 and 5. a) log(0.4)~...
-
Using the information that follows, compute the standard unit cost of product MZW: Direct materials quantity standard...... 5 pounds per unit Direct materials price standard........ $10.20 per pound...
-
A random variable, X, has a Gaussian PDF with mean 5 and unit variance. We measure 10 independent samples of the random variable. (a) Determine the expected value of the sample mean. (b) Determine...
-
14. Using the assumptions of Example 4, and the stock price derived in Example 5 suppose you were to perform a naive valuation of the convertible as a risk-free bond plus 50 call options on the...
-
Assume that a salesperson, Edwynn Phillips, has the following annual compensation package: C = $15,000 + 0.2(own sales) This compensation plan induces Ed to exert a given level of effort in selling....
-
Batu Ltd operates in Windhoek, manufacturing sandals. The company uses a standard costing system, with a plan to produce 10,000 pairs of sandals each month this year. The following standard costs...
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq) + 1(s) 2+ b. SO32 (aq) + MnO4 (aq) SO42 (aq) + Mn+ (aq) 2- c. S03 (aq) + Cl(g) SO4 (aq) + Cl (aq)
-
On aging for longer times, why do the second-phase precipitates grow? What is the driving force? Compare this with driving forces for grain growth and solid-state sintering.
-
do you agree wih this approach to dismantling the toxic culture? explain
-
Movies When randomly selecting a speaking character in a movie, the probability of getting a female is 0.331 (based on data from "Inequality in 1200 Popular Films," by Smith, et al., Annenberg...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob O'Donnell, a local merchant, to contribute the capital to form a partnership....
-
Exercise 6-10A (Algo) Double-declining-balance and units-of-production depreciation: gain or loss on disposal LO 6-3, 6-4, 6-5 Exact Photo Service purchased a new color printer at the beginning of...
-
Independent Events Again assume that when randomly selecting a speaking character in a movie, the probability of getting a female is 0.331, as in Exercise 1. If we want to find the probability of 20...
-
On January 1, 2016. Metco. Inc.. reported 822.100 Problem 8.26 shares of $5 par value common stock as being issued and outstanding. On March 15, 2016. Metco. Inc.. purchased for its treasury 7,200...
-
A firm has the following balance sheet: Assets Cash Accounts receivable Inventory Plant and equipment $ 15,000 150,000 92,000 170,000 $427,000 Liabilities and Equity Accounts payable Long-term debt...
-
Consider a swimming pool and a tall graduated cylinder (like the one in Fig. Q10.4), both containing water and filled to the same height. (a) Is the pressure at the bottom of the pool greater than,...
-
A block sits on a plate that in turn rests on the surface of a fluid as shown in Figure P10.11. The plate is partially submerged in the fluid, its area is 0.30 m 2 , and there is air at atmospheric...
-
Why are dams thicker at the bottom than at the top?
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


