Balance each redox reaction occurring in acidic aqueous solution. a. I (aq) + NO (aq) b. CIO4
Question:
Balance each redox reaction occurring in acidic aqueous solution.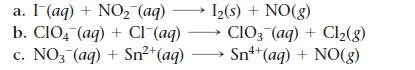
Transcribed Image Text:
a. I (aq) + NO₂ (aq) b. CIO4 (aq) + Cl¯(aq) c. NO3(aq) + Sn²+ (aq) 1₂(s) + NO(g) CIO3(aq) + Cl₂(g) Sn+ (aq) + NO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
a Iaq NOaq Is NOg Halfreactions Oxidation Iaq Is 2e Reduction NOaq Haq e NOg HOl Balance the oxidati...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PART A: Balance each redox reaction occurring in acidic aqueous solution. MnO4( a q )+Al( s )Mn2+( a q )+Al3+( a q ). Express your answer as a chemical equation. Identify all of the phases in your...
-
Balance each redox reaction occurring in acidic aqueous solution. a. PbO(s) + I (aq) Pb+ (aq) + 1(s) 2+ b. SO32 (aq) + MnO4 (aq) SO42 (aq) + Mn+ (aq) 2- c. S03 (aq) + Cl(g) SO4 (aq) + Cl (aq)
-
Balance each redox reaction occurring in acidic aqueous solution. a. Zn(s) + Sn+ (aq) b. Mg(s) + Cr+ (aq) Zn+ (aq) + Sn(s) Mg+ (aq) + Cr(s) c. MnO4 (aq) + Al(s) Mn+ (aq) + A1+ (aq)
-
You require inventory and accounts receivable collateral for all C&I loans. You have a guideline of an advance rate of 70% for customer receivables of less than 60 days of age. Older receivables get...
-
Lago Corporation is considering adopting the standard costing method. Dan Sarkis, the manager of the Ohio Division, attended a corporate meeting at which Leah Rohr, the controller, discussed the...
-
Show that if Xn ,n = 1, 2, 3, is a sequence of IID Gaussian random variables, the sample mean and sample variance are statistically independent.
-
16. As discussed in the text, compensation options are prematurely exercised or canceled for a variety of reasons. Suppose that compensation options both vest and expire in 3 years and that the...
-
On January 1, 2017, Spring Fashions Inc. enters into a contract with a southeast retail company to provide 500 dresses for $ 62,500 ($ 125 per dress) over the next 10 months. On October 1, 2017,...
-
Q. What is the effect of the 2018 Tax Cuts & Jobs Act on standard deduction and itemized deduction?
-
Balance each redox reaction occurring in basic aqueous solution. a. HO(aq) + ClO(aq) CIO (aq) + O(g) b. Al(s) + MnO4 (aq) MnO(s) + Al(OH)4 (aq) c. Cl(g) Cl(aq) + CIO (aq)
-
How can the corrosion of iron be prevented?
-
Refer to Exercise 12. Assume that the cost of an engine repair is $50, and the cost of a transmission repair is $100. Let T represent the total cost of repairs during a one-hour time interval. a....
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Identifying Binomial Distributions. Determine whether the given procedure results in a binomial distribution or a distribution that can be treated as binomial (by applying the 5% guideline for...
-
Case 6: TOMS Shoes in 2016: An Ongoing Dedication to Social Responsibility, by Margaret A. Peteraf, Sean Zhand, and Meghan L. Cooney (page C-57) Read the case and then respond to the case questions...
-
Quatro Co. issues bonds dated January 1, 2019, with a par value of $740,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
Wildcat Mining wants to know the appropriate discount rate to use in their capital budgeting decision making process. Based on the following data, what is the weighted average cost of capital the CFO...
-
Permabilt Corp. was incorporated on January 1, 2016, and issued the following stock for cask 4,000,000 shares of no-par common stock were authorized; 1,750,000 shares were issued on January 1, 2016....
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
Estimate the pressure exerted by your feet when you stand upright on the floor. Compare it to the pressure when you are wearing stiletto heels (use the value at the heel).
-
When traveling in a commercial airplane, you are sometimes given snacks, such as peanuts, in a sealed bag. The bag is often bulging much more than when you purchase a bag of peanuts in a store....
-
When a typical 1200-page textbook is sitting on a table, it exerts a force on the table. Estimate the associated pressure.
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


