Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction
Question:
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.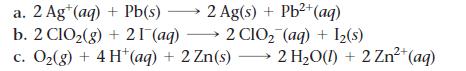
Transcribed Image Text:
a. 2 Ag+ (aq) + Pb(s) → 2 Ag(s) + Pb²+ (aq) b. 2 C1O₂(g) + 21 (aq) 2 CIO₂ (aq) + 1₂(s) c. O₂(g) + 4H+ (aq) + 2 Zn(s) → 2 H₂O(l) + 2 Zn²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
4 Anode Pb Pb Pbs 2 Pb aq 2 e Salt bridge Ag A...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Use line notation to represent each electrochemical cell in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Use line notation to represent each electrochemical cell in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Determine whether the given functions are even, odd, or neither. a. x sin(x) c. x cos(x) e. x sin(x) + x sin(x) f. x sin(x) + x cos(x) even h. x sin(x) + x cos(x) j. x cos (x) + x cos(x) b. x sin(x)...
-
Garden Metal Works produces lawn sculptures. The company analyzes only variances that differ by more than 5 percent from the standard cost. The controller computed the following direct labor...
-
Let the random variables U, V, and W be as described. (a) Find the MAP estimator of U given the observation {V = v, W = w}. (b) Find the ML estimator of U given the observation {V = v, W =w}. (c)...
-
13. Suppose a firm has 20 shares of equity and a 10-year zero-coupon convertible bond with a maturity value of $200, convertible into 8 shares. What is the value of the debt, the share price, and the...
-
Diamond Software, Inc., does software development. One important activity in software development is writing software code. The manager of the Wordpro Development Team determined that the average...
-
The Maitland Furniture store gets an average of 50 customers per shift. The manager of Maitland wants to calculate whether she should hire 1, 2, 3, or 4 salespeople. She has determined that average...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O(g) Ag(CN) (aq) c. NO (aq) + Al(s) - NH3(g) + AlO (aq)
-
Shaft graves in ancient Greece. Refer to the American Journal of Archaeology (Jan. 2014) study of sword shaft graves in ancient Greece, Exercise 2.37 (p. 78). The number of sword shafts buried at...
-
Complete the "Leadership Vision Questionnaire" in Chapter 7 (p176). Reflect on your results and complete the following prompts: Share the results from your questionnaire. Be sure to include the final...
-
1. Prepare el Presupuesto Operacional hasta completar el COGS (70 puntos) La empresa ACCO 295 tiene una venta proyectada de $450,000 Cada unidad se vende $450 Su inventario inicial 300 (costo $125)...
-
Continuing Case 65. Retirement Income Forecast Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that...
-
The partnership of Frick, Wilson, and Clarke has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the following account balances: Cash...
-
Harry and Sally went to a large hardware store and told the salesperson they wanted the cheapest rotating clothesline in stock, provided it would bear a heavy load of washing. The salesperson assured...
-
Enter the following column headings across the top of a sheet of paper: Enter the transaction letter in the first column and show the effect (if any) of each of the following transactions on each...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Consider a box with a lid 1.5 m wide and 0.70 m long. If the inside of the box is evacuated (i.e., its pressure is zero), how much force is required to open the lid? Could you open the lid?
-
Why does the lava in a Lavalamp (Fig. Q10.10) rise and then fall? Figure Q10.10
-
The pressure in the atmosphere is not constant, but fluctuates as the weather changes. If the pressure outside a window drops by 5% while the pressure inside does not change, what is the force on the...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


