Calculate the standard cell potential for each of the electrochemical cells in Problem 43. Problem 43 Sketch
Question:
Calculate the standard cell potential for each of the electrochemical cells in Problem 43.
Problem 43
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.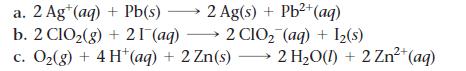
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





