Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction
Question:
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the direction of electron flow.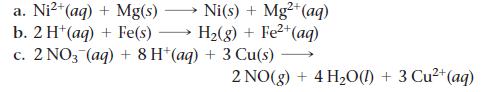
Transcribed Image Text:
2+ Ni(s) + Mg²+ (aq) H₂(g) + Fe²+ (aq) a. Ni²+ (aq) + Mg(s) b. 2 H+ (aq) + Fe(s) c. 2 NO3(aq) + 8 H+ (aq) + 3 Cu(s) 2 NO(g) + 4H₂O(l) + 3 Cu²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Redox Reaction Niaq Mgs Nis Mgaq Voltaic Cell Anode Oxid...View the full answer

Answered By

Jehal Shah
I believe everyone should try to be strong at logic and have good reading habit. Because If you possess these two skills, no matter what difficult situation is, you will definitely find a perfect solution out of it. While logical ability gives you to understand complex problems and concepts quite easily, reading habit gives you an open mind and holistic approach to see much bigger picture.
So guys, I always try to explain any concept keeping these two points in my mind. So that you will never forget any more importantly get bored.
Last but not the least, I am finance enthusiast. Big fan of Warren buffet for long term focus investing approach. On the same side derivatives is the segment I possess expertise.
If you have any finacne related doubt, do reach me out.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that occurs at each electrode and the species present in each solution. Also indicate the...
-
Use line notation to represent each electrochemical cell in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Use line notation to represent each electrochemical cell in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Landry State University, a public university located in Louisiana, has a June 30 fiscal year. On July 20 of the current year, Landry State University receives $2,125,000 in payments from the U.S....
-
Prepare a flexible budget for 10,000, 12,000, and 14,000 units of output, using the following information: Variable costs Direct materials.......$10.00 per unit Direct labor...........$3.00 per unit...
-
Let the random variables U and V be as described. (a) Find the MAP estimator of U given the observation V = v. (b) Find the ML estimator of U given the observation V= v. (c) Find the LMMSE estimator...
-
12. Suppose a firm has 20 shares of equity, a 10-year zero-coupon debt with a maturity value of \($200\), and warrants for 8 shares with a strike price of \($25\). What is the value of the debt, the...
-
Danas Ribbon World makes award rosettes. Following is information about the company: Variable cost per rosette ...... $ 1.60 Sales price per rosette ...... 3.00 Total fixed costs per month .......
-
1. Dividend income for FVOCI should be recognized in the Statement of Other comprehensive income. True or False? 2. Fair Value through Profit or Loss equity Securities or Trading securities are...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
Balance each redox reaction occurring in basic aqueous solution. a. MnO4 (aq) + Br (aq) MnO(s) + BrO3(aq) b. Ag(s) + CN- (aq) + O(g) Ag(CN) (aq) c. NO (aq) + Al(s) - NH3(g) + AlO (aq)
-
What are the critical activities or tasks in the projectthat is, which activities will delay the entire project if they are late? lop5
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Payroll Payroll Register Register Thomas Avery Towle...
-
Name: Course: Worksheet Lab Experience 5 Logic Circuits (A) Exercise 5.1 Truth table for the example circuit A B Output Value 0 0 1 1 0 1 1 Exercise 5.2 A slight change in the example circuit...
-
Stanley Medical Hospital is a non-profit and a non-chartered hospital planning to acquire several hospitals in the area. The hospital is researching financial options since they want to expand into...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2022, for $12,000. They expect to use the...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
Enter the following column headings across the top of a sheet of paper: Enter the transaction letter in the first column and show the effect (if any) of each of the following transactions on each...
-
What recommendations would you make to Big Four firms to help them (1) avoid confrontations with governmental officials in an authoritarian society and (2) deal effectively with such confrontations...
-
A cargo ship travels from the Mississippi River into the Gulf of Mexico. Will the ship sink or rise with respect to the waterline as it moves from the river to ocean water? Why?
-
Consider an airplane window (area 0.30 m 2 ). If the pressure inside the plane is atmospheric pressure and the pressure outside is 20% of P atm , what is the force on the window? Ignore the velocity...
-
Three containers are filled with water to the same height (Fig. Q10.9). For which container is the pressure at the bottom the greatest? Or, are the pressures the same? Explain. Figure Q10.9 Container...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


