Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the
Question:
Consider the voltaic cell: 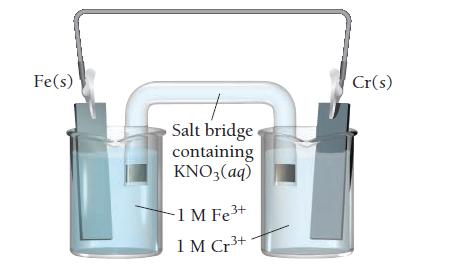
a. Determine the direction of electron flow and label the anode and the cathode.
b. Write a balanced equation for the overall reaction and calculate E°cell.
c. Label each electrode as negative or positive.
d. Indicate the direction of anion and cation flow in the salt bridge.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





