Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the
Question:
Consider the voltaic cell:
a. Determine the direction of electron flow and label the anode and the cathode.
b. Write a balanced equation for the overall reaction and calculate E°cell.
c. Label each electrode as negative or positive.
d. Indicate the direction of anion and cation flow in the salt bridge.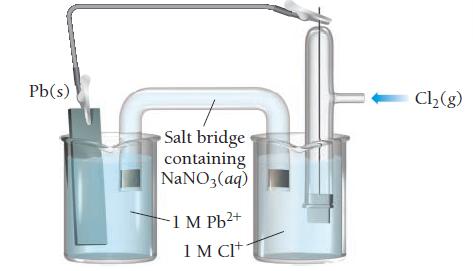
Transcribed Image Text:
Pb(s) Salt bridge containing NaNO3(aq) -1 M Pb²+ 1 M CI+ Cl₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To answer your questions about the voltaic cell in the image provided Ill go through each point one by one a Determine the direction of electron flow ...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the cathode. b. Write a balanced equation for the overall reaction and calculate E cell . c. Label each...
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
26. Originally from England, Joy received her permanent Canadian resident status three years ago. She lives in Edmonton, where she works as a surgeon major hospital. She travels back to her home...
-
What is the timestep value ? And how do I go about altering from downstream to upstream? The following code solves the advection equation 1 2 3 4 5 6 7 8 9- 10 - 11 12 - 13 - 14 - 15 - 16 - 17 18 19...
-
To the Point Manufacturing Company uses the standard costing method. The companys main product is a fine-quality fountain pen that normally takes 2.5 hours to produce. Normal annual capacity is...
-
Repeat exercise 5.66 Suppose In figure 5.7 and P i = 1/3, i = 1, 2, 3. Determine the mutual information for this channel. If Can you give an interpretation for your result. 0.8 0.1 0.1 Q = 10.1 0.8...
-
8. Nowsuppose the firm finances the project by issuing debt that has lower priority than existing debt. How much must a \($1\), \($10\), or \($25\) project be worth if the shareholders are willing to...
-
"I know headquarters wants us to add that new product line," said Dell Havasi, manager of Billings Company's Office Products Division. "But I want to see the numbers before I make any move. Our...
-
SkyChefs, Inc., prepares in-flight meals for a number of major airlines. One of the company's products is grilled salmon in dill sauce with baby new potatoes and spring vegetables. During the most...
-
Use line notation to represent each electrochemical cell in Problem 43. Problem 43 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the half-reaction that...
-
Calculate the standard cell potential for each of the electrochemical cells in Problem 44. Problem 44 Sketch a voltaic cell for each redox reaction. Label the anode and cathode and indicate the...
-
Fill in the blank(s) to correctly complete the sentence. For the following sum to be true, we must have w = ______, x = ______, y = _______, and z = _______. 5 | 1 Z. -3 10 4. || 2.
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Bacon, Inc.. has the following stockholders' equity section in its May 31, 2016, comparative balance sheets: Required: a. Calculate the amount that should be shown on the balance sheet for common...
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
Explain how Archimedess principle can be used to measure a persons percentage body fat. Fat has a different density than other body tissue.
-
The specific gravity of a substance is the ratio of its density to the density of water. Among other applications, measurements of the specific gravity are used by mineralogists to determine the...
-
In a neutron star, all the mass has collapsed into a relatively small volume. (a) If a particular neutron star has a radius of 1000 m and a mass of 2.0 10 28 kg, what is its density? (b) Compare...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


