Based on these molecular views, determine whether each pictured acid is weak or strong. H3O+- HF- F
Question:
Based on these molecular views, determine whether each pictured acid is weak or strong.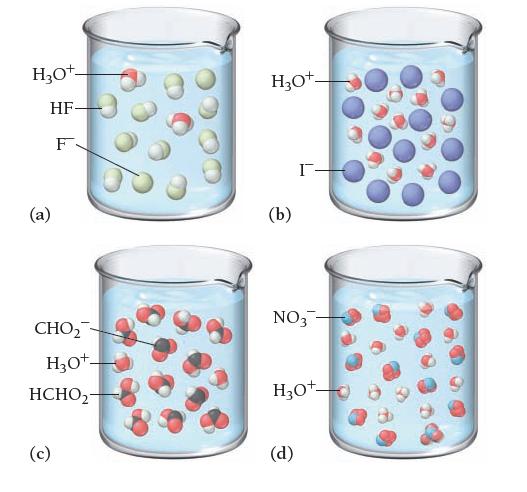
Transcribed Image Text:
H3O+- HF- F (a) CHO₂ H3O+- HCHO2- (c) H₂O+- (b) I- NO3- H₂O+ (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Weak ...View the full answer

Answered By

Michael Mulupi
I am honest,hardworking, and determined writer
4.70+
72+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Based on these molecular views, determine whether each pictured base is weak or strong. NH3- NH4+ (a) H2CO3- - Nat- HCO3- (c) OH Nat- (b) OH- Sr2+_ (d)
-
Disc jockey Lee Jason Kibler uses turntables and other performers' vocals to produce music containing jazz and funk elements. Since 1999, he has performed and released several albums under the name...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Describe the three ways a client can reference a name from a namespace in C++.
-
What is the cause of an unfavorable volume variance? Does the volume variance convey any meaningful information to managers?
-
Explain asymmetric information, the problems it causes, and solutions to these problems.
-
2. Follow the instructions at the end of the case.
-
Crosser Company budgets on a quarterly basis. The following beginning and ending inventory levels (in units) are planned for the first and second quarters of 2009. Required If Crosser Company were to...
-
(20 points) Consider the binomial tree model. Suppose the stock price at time zero is So, and the stock does not pay dividends. The risk free rate is r and the time step is At. Consider an exotic...
-
The binding of oxygen by hemoglobin in the blood involves the equilibrium reaction: In this equation, Hb is hemoglobin. The pH of normal human blood is highly controlled within a range of 7.35 to...
-
Identify the Lewis acid and Lewis base from among the reactants in each equation. a. Ag (aq) + 2NH3(aq) Ag(NH3)2+(aq) b. AlBr3 + NH3 H3NAIBr3 C. F (aq) + BF3(aq) = BF4 (aq)
-
For several years, evidence had been mounting that folic acid reduces major birth defects. A. Czeizel and I. Dudas of the National Institute of Hygiene in Budapest directed a study that provided the...
-
Sometimes when we are asked for a linear model, the information that we are given is data about a scenario. In these cases we have to use Excel to generate a trendline. There is a video in this...
-
1. Purpose Explain 3 points from the Introduction section as to why this study is important. How did this study build on the existing literature in this area? 2. Participants Outline at least 2...
-
In this Capstone experience, you will develop a strategy playbook for a selected organization. You may be familiar with the concept of a playbook as it relates to a sports team, but what might that...
-
On January 1, 2024, the general ledger of Big Blast Fireworks includes the following account balances: Accounts Cash Debit Credit $25,900 Accounts Receivable 46,500 Allowance for Uncollectible...
-
The WRX can travel 1 / 4 of a mile in 1 3 . 9 sec . Calculate the acceleration over this distance if assumed constant.
-
Consider two random variables X and Y. Suppose that Y takes on k values y1, . . . , yk and that X takes on / values x1, . . . , xl. (b) Use your answer to (a) to verify Equation (2.19). (c) Suppose...
-
Determine by direct integration the values of x for the two volumes obtained by passing a vertical cutting plane through the given shape of Fig. 5.21. The cutting plane is parallel to the base of the...
-
Saturn makes one complete orbit of the Sun every 29.4 years. Calculate the radius of the orbit of Saturn.
-
In Section 5.4, we showed that the radius of a geosynchronous orbit about the Earth is 4.2 107 m, compared with the radius of the Earth, which is 6.4 106 m. By what factor is the force of gravity...
-
You are an astronaut (m = 95 kg) and travel to a planet that is the same radius and mass as the Earth, but it has a rotational period of only 2 h. What is your apparent weight at the equator of this...
-
You are considering investing in a no-load mutual fund with an annual expense ratio of .6% and an annual 12b-1 fee of .75%. You could also invest in a bank CD paying 6.5% per year. What minimum...
-
Finanz Inc. is a firm located in Zuerich, Switzerland. On July 1, the firm paid CHF 14,000 to its landlord for 4 months rent beginning July 1. Prepaid Rent was debited for the full amount. The firm...
-
You are trying to plan for retirement in 20 years by depositing $10,000 every 6 months in your retirement savings account for the next 25 years, until retirement. Assume that the account returns 5%...

Study smarter with the SolutionInn App


