Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written.
Question:
Calculate E°cell for each balanced redox reaction and determine if the reaction is spontaneous as written.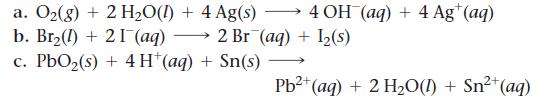
Transcribed Image Text:
a. O₂(g) + 2 H₂O(1) + 4 Ag(s) b. Br₂(1) 21 (aq) c. PbO₂ (s) + 4H+ (aq) + Sn(s) 4 OH(aq) + 4 Ag+ (aq) 2 Br (aq) + 1₂(s) 2+ Pb²+ (aq) + 2 H₂O(1) + Sn²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Reaction Ecell V Spontaneous O2g 2 H2Ol 4 Ags 4 OHaq 4 Agaq 0000 ...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written. 2+ a. 2 Cu(s) + Mn+ (aq) b. MnO(aq) + 4H+ (aq) + Zn(s) c. Cl(g) + 2 F (aq) 2 Cut (aq) +...
-
Toplob Inc. ("Toplob") provides employment consulting services. These services range from maintaining payroll records to taxation services, as well as general business advisory and consulting to new...
-
What is the importance of developing plans to achieve organizational goals? explain with examples
-
Design Architects, LLP, billed clients for 6,000 hours of design work for the month. Actual variable overhead costs for the month were $315,000, and 6,250 hours were worked. At the beginning of the...
-
An experiment consists of selecting a point from the interior of the unit circle, x2 + y2 <1. (a) What fraction of the points in the space satisfy x > 1/2? (b) What fraction of the points in the...
-
19. This problem has been intentionally omitted for this edition.
-
a. Assuming that investments A and B are equally risky and using the 4% discount rate, apply the present value technique to assess the acceptability of each investment and to determine the preferred...
-
Determine Sheffield's variable - MOH price and efficiency variances. Also identify whether the company's variable - MOH costs were under - or overapplied, and by how much. Variable - MOH price...
-
Which metal cation is the best oxidizing agent? a. Pb+ b. Cr+ c. Fe+ d. Sn+
-
Determine whether or not each metal dissolves in 1 M HIO 3 . For those metals that do dissolve, write a balanced redox equation for the reaction that occurs. a. Au b. Cr
-
Access the Object Database Management Group Web site (www.odmg.org) and gather information on the current status of the ODMG standard.
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
The following information is available from Bromfield Co.'s accounting records for the year ended December 31, 2016 (amounts in millions): Cash dividends declared and...
-
Find the work done in pumping all the oil (density S = 50 pounds per cubic foot) over the edge of a cylindrical tank that stands on one of its bases. Assume that the radius of the base is 4 feet, the...
-
Figure P.11.25 depicts a single saw tooth function and its convolution. Note that the convolution is asymmetricalexplain why thats reasonable. Why does the convolution begin at 0? How wide is the...
-
Graphically convolve the two functions Æ(x) and h(x) shown in Fig. P.11.26. Fig. P.11.26. How wide will the convolution be? Will it be symmetrical? Where will it start? h(x) f(x) 1 +1 -2 +2 -1
-
Graphically convolve, at least approximately, the two functions shown in Fig. P.11.31. Does that solution remind you of anything? Why is the convolution symmetrical? When does its peak value occur in...
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


