Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written.
Question:
Calculate E°cell for each balanced redox reaction and determine if the reaction is spontaneous as written.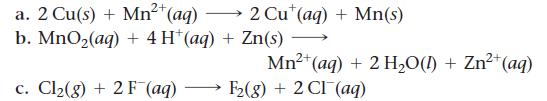
Transcribed Image Text:
2+ a. 2 Cu(s) + Mn²+ (aq) b. MnO₂(aq) + 4H+ (aq) + Zn(s) c. Cl₂(g) + 2 F (aq) 2 Cut (aq) + Mn(s) 2+ Mn²+ (aq) + 2 H₂O(1) + Zn²+ (aq) F₂(g) + 2 CI (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 170 V nonsp...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate E cell for each balanced redox reaction and determine if the reaction is spontaneous as written. a. O(g) + 2 HO(1) + 4 Ag(s) b. Br(1) 21 (aq) c. PbO (s) + 4H+ (aq) + Sn(s) 4 OH(aq) + 4 Ag+...
-
Toplob Inc. ("Toplob") provides employment consulting services. These services range from maintaining payroll records to taxation services, as well as general business advisory and consulting to new...
-
Which 3 accounts can't be merged in the chart of accounts?
-
At the beginning of last year, Diekow Productions set variable overhead standards of 10 machine hours at a rate of $ 10 per hour for each product produced. During the year, 10,800 machine hours were...
-
An Erlang Random variable has a PDF of the form (a) Find the characteristic function, X( ) . (b) Find the Taylor series expansion of X( ) . (c) Find a general expression for the k th moment of X....
-
21. A DECS contract pays two shares if ST < 27.875, 1.667 shares if the price is above ST > 33.45, and \($27.875\) and \($55.75\) otherwise. The quarterly dividend is $0.87. Value this DECS assuming...
-
AAA Appliances Inc. has two production departments. The nature of the process is such that no units remain in process in Finishing at the end of the period. At the beginning of the period, 10,000...
-
A stock trades for $42 per share. A call option on that stock has a strike price of $5 and an expiration date nine months in the future. The volatility of the stock's returns is 35%, and the...
-
Determine whether or not each metal dissolves in 1 M HIO 3 . For those metals that do dissolve, write a balanced redox equation for the reaction that occurs. a. Au b. Cr
-
Determine whether or not each metal dissolves in 1 M HNO 3 . For those metals that do dissolve, write a balanced redox reaction showing what happens when the metal dissolves. a. Cu b. Au
-
Nitroglycerin is a shock-sensitive liquid that detonates by the reaction Calculate the total volume of product gases at 88.5 kPa and 175C from the detonation of 1.00 lb (454 g) of nitroglycerin. 4...
-
3. Suppose we have n i.i.d., uniform-(0,t) random variables. Place these random variables on the interval (0, t]. Let 0 = 80 < 81 < ... < Sn1 < (0,t]. Skt. Compute the probability that there are in...
-
3. (3 pts) Use Python to write a function that takes a single input, a list of numbers. The function should loop through the list and, on each iteration, print the number if it is the largest number...
-
a) A linear charge density = 4z C/m is distributed on the z axis, what is the total charge within a cylinder of radius r = 0.5 m and height h = 5 m which extends from z = 1 to z = 4? b) A uniform...
-
Read the articles given below on module 9 now read the articles given below on module 10 Now answer these questions based on both modules slideshow pictures and the links readings Describe how the...
-
2. For each equation, state the quantity (with units) represented by each variable. a) D=mV F b) P== A c) P = Dgh g 3. Write a single sentence answering each question. a) If the mass remains constant...
-
The following information is avail-able from the accounting records of Spenser Co. for the year ended December 31, 2016: Selling, general, and administrative...
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Imagine that we have Youngs Experiment, where one of the two pinholes is now covered by a neutral-density filter that cuts the irradiance by a factor of 10, and the other hole is covered by a...
-
Show (for normally incident plane waves) that if an aperture has a center of symmetry (i.e., if the aperture function is even), then the diffracted field in the Fraunhofer case also possesses a...
-
Suppose a given aperture produces a Fraunhofer field pattern E(Y, Z). Show that if the apertures dimensions are altered such that the aperture function goes from A(y, z) to A(αy,...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


