Calculate H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline,
Question:
Calculate ΔHrxn for the combustion of octane (C8H18), a component of gasoline, by using average bond energies and then calculate it using enthalpies of formation from Appendix IIB.
What is the percent difference between your results? Which result would you expect to be more accurate?
Appendix IIB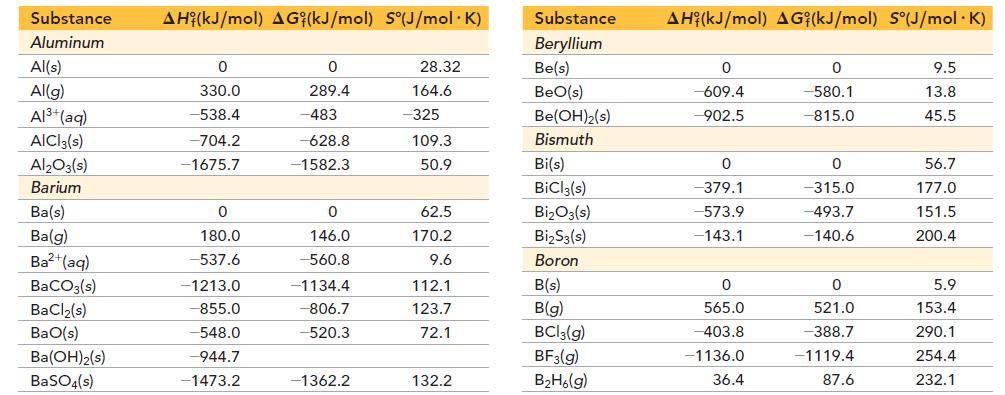
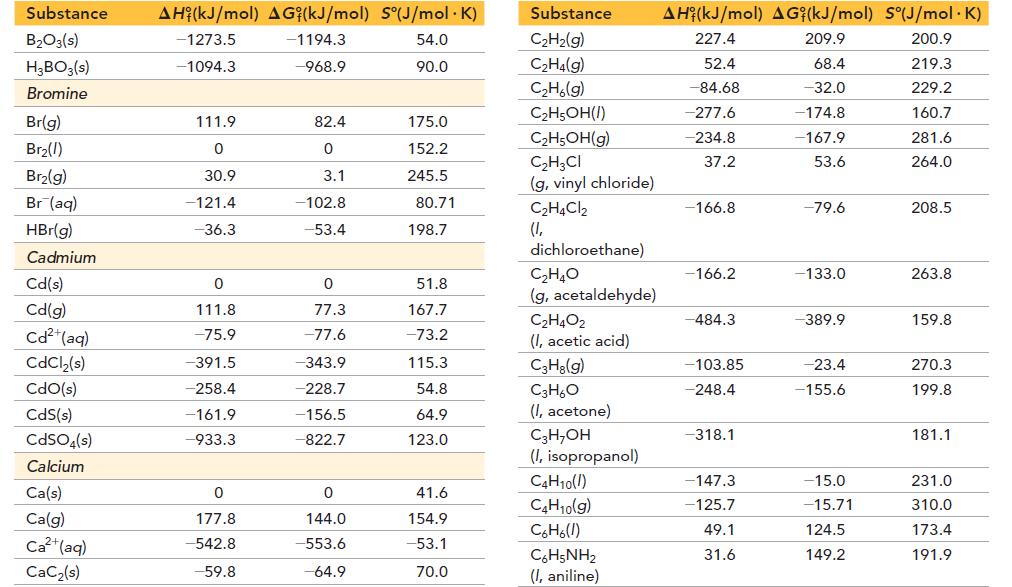
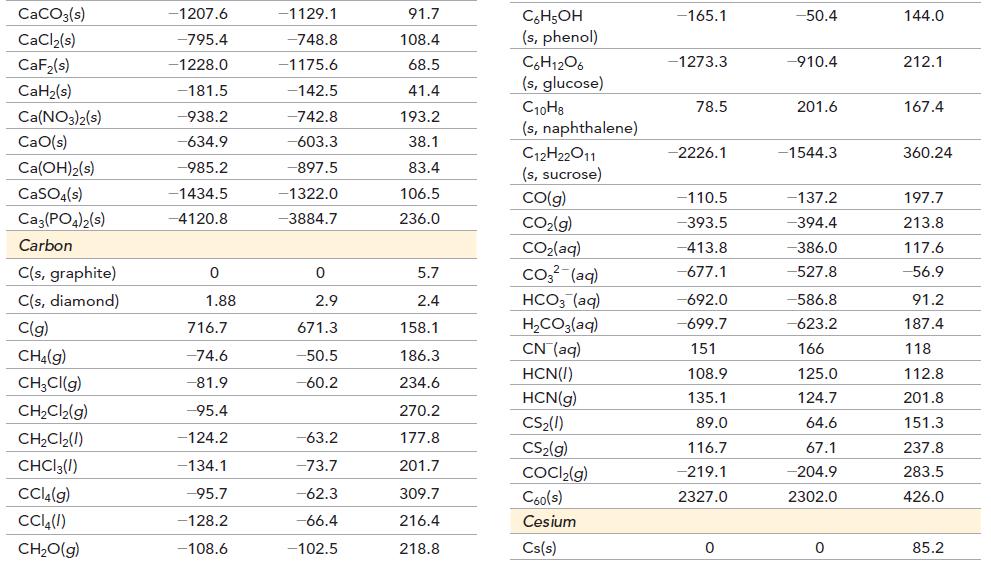
Transcribed Image Text:
Substance Aluminum Al(s) Al(g) Al³+(aq) AlCl3(s) Al₂O3(s) Barium Ba(s) Ba(g) Ba²+(aq) BaCO3(s) BaCl₂(s) BaO(s) Ba(OH)₂(s) BaSO4(s) AH (kJ/mol) AG (kJ/mol) S°(J/mol. K) 0 330.0 -538.4 -704.2 -1675.7 0 180.0 -537.6 -1213.0 -855.0 -548.0 -944.7 -1473.2 0 289.4 -483 -628.8 -1582.3 0 146.0 -560.8 -1134.4 -806.7 520.3 -1362.2 28.32 164.6 -325 109.3 50.9 62.5 170.2 9.6 112.1 123.7 72.1 132.2 Substance Beryllium Be(s) BeO(s) Be (OH)2(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Bi₂S3(s) Boron B(s) B(g) BCI 3(g) BF3(g) B₂H6(g) AH (kJ/mol) AG (kJ/mol) S°(J/mol. K) 0 -609.4 -902.5 0 -379.1 -573.9 -143.1 0 565.0 -403.8 -1136.0 36.4 0 -580.1 -815.0 0 -315.0 -493.7 -140.6 0 521.0 -388.7 -1119.4 87.6 9.5 13.8 45.5 56.7 177.0 151.5 200.4 5.9 153.4 290.1 254.4 232.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Average bond energies and enthalpies of formation are the two approaches we can use to compute Hrxn ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate ? H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline, by using average bond energies. Calculate ? H rxn for the combustion of octane by using enthalpies of formation...
-
A city of 100,000 people uses approximately 1.0 * 10 11 kJ of energy per day. Suppose all of that energy comes from the combustion of liquid octane (C 8 H 18 ) to form gaseous water and gaseous...
-
It is desired to control the amount of CO in the products of combustion of octane C8H18 so that the volume fraction of CO in the products is less than 0.1 percent. Determine the percent theoretical...
-
Be able to explain how land policy shaped economic growth in the United States.
-
The BSN Company would like a hard copy report of all the current vendors in its database. Figure provides a suggested format for the report. Note that your report header should include the company...
-
Soical Media Influencers Celebritieshaveendorsedproductsinadvertisingandpromotionalcampaignsfordecades.In recenttimes,socialmediapersonalitiesoftentrytoinfluencepotentialcustomers.Thekey...
-
In which part of your field do you consider yourself to be an expert?
-
Jim Reed manages a fleet of utility trucks for a rural county government. Hes been in his job 30 years, and he knows where the angles are. He makes sure that when new trucks are purchased, the...
-
Question 25 4 pts Biscuits Inc. has offered $429 million cash for all of the common stock in Gravy Corporation. Based on recent market information, Gravy is worth $380 million as an independent...
-
A steel block with a density of 7800 kg/m 3 is suspended from a string in a beaker of water so that the block is completely submerged but not resting on the bottom. The block is a cube with sides of...
-
Draw the Lewis structure for urea, H 2 NCONH 2 , one of the compounds responsible for the smell of urine. (The central carbon atom is bonded to both nitrogen atoms and to the oxygen atom.) Does urea...
-
Draw the Lewis structure for HCSNH 2 . (The carbon and nitrogen atoms are bonded together, and the sulfur atom is bonded to the carbon atom.) Label each bond in the molecule as polar or nonpolar.
-
For the beam considered in Problem 12.8 (Fig. 12.20), derive the assembled stiffness and mass matrices of the system. Data From Problem 12.8:- For the beam shown in Fig. 12.20, one end (point \(A\) )...
-
Skinovations needs to put together a Production schedule for next week and has asked its marketing team to give its forecasts for next week's sales. The team has used two different forecasting...
-
If a potential leader viewed her least preferred co-worker in favorable terms, how would Fiedler's Model describes this leader?
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company s financial statements,...
-
For our first discussion you should locate a research article in which a quantitative study is reported. This article should not be a theoretical article or a methods article, but should describe...
-
A box is separated by a partition which divides its volume in the ration of 3:1. the larger portion of the box contains 1000 molecules of Ne gas; the smalled portion contains 100 molecules of He gas....
-
Sometimes cos cos in Equation 7.2 is termed the Schmid factor. Determine the magnitude of the Schmid factor for an FCC single crystal oriented with its [100] direction parallel to the loading axis.
-
Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+ (b) p3- (c) Zr4+ (d) Ru3+ (e) As3- (f) Ag+
-
Consider the motion of a marble as it falls to the bottom of a jar of honey. Experiments show (see also Chapter 3) that the marble moves with a constant velocity. Applying Newtons first law, does...
-
Figure P2.17 shows several hypothetical velocitytime graphs. For each case, sketch qualitatively the corresponding accelerationtime graph. Figure P2.17 Case 2 Case 1 Case 3
-
Consider the motion of the Moon as it orbits the Earth. (a) Is the Moons acceleration zero or nonzero? Explain. (b) If the Moon has a nonzero acceleration, what force is responsible?
-
S Corporation is expanding rapidly and it currently needs to retain all of its earnings. Hence, it does not pay any dividends. However, investors expect S Corp to begin paying dividends with the...
-
What is law accordingly to John Austin ? In what sense laws are different from morality? Discuss the importance of knowing commercial law for business executives.
-
Joey purchased a 14-year T-bond with a 3.5% annual coupon four years ago at par. Today the bond's YTM 5%. If Cramer holds this bond to maturity, what internal rate of return will he earn on this...

Study smarter with the SolutionInn App


