Calculate the equilibrium constant for each of the reactions in Problem 66. Problem 66 Use tabulated electrode
Question:
Calculate the equilibrium constant for each of the reactions in Problem 66.
Problem 66
Use tabulated electrode potentials to calculate ΔG°rxn for each reaction at 25 °C.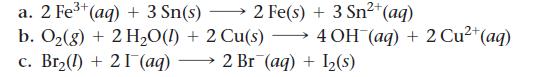
Transcribed Image Text:
2+ a. 2 Fe³+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn²+ (aq) b. O₂(g) + 2 H₂O(l) + 2 Cu(s) →→→ 4 OH(aq) + 2 Cu²+ (aq) c. Br₂(1) + 21 (aq) 2 Br (aq) + 1₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The equilibrium constant K for a chemical reaction is related to the standard free energy change Grxn for the reaction by the following equation Grxn ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant for each of the reactions in Problem 65. Problem 65 Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2...
-
Calculate the equilibrium constant for each of the reactions at25 ?C. Standard Electrode Potentials at 25 ?C Reduction Half-Reaction E ?(V) Pb2+( a q )+2 e ? ?Pb( s ) -0.13 Mg2+( a q )+2 e ? ?Mg( s )...
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn+ (aq) b. O(g) + 2 HO(l) + 2 Cu(s) 4 OH(aq) + 2 Cu+ (aq) c. Br(1) + 21 (aq)...
-
A financial institution can borrow $100 million for 2 years at 3%. It plans to invest this money in a 1-year security with an interest rate of 4.8% per year. Calculate net interest income for the...
-
Home Products Company manufactures a complete line of kitchen glassware. The Beverage Division specializes in 12-ounce drinking glasses. Erin Fisher, the superintendent of the Beverage Division,...
-
a. What is a process? b. What is the relationship between a process and its subprocesses? c. What is statistical process control?
-
13. Use the information in Table 5. a. What is the price of a bond that pays one barrel of oil 2 years from now? b. What annual cash payment would the bond have to make in order to sell for $20.90?
-
Sound Investments, Inc. is a large retailer of stores equipment. The controller is about to prepare the budget for the first quarter of 20x2. Past experience has indicated that 75 percent of the...
-
What does the cost of capital represent? What is the significance of the cost of capital in the financial manager's primary goal of maximizing shareholder wealth? In other words, how does the...
-
Calculate the equilibrium constant for the reaction between Ni 2+ (aq) and Cd(s) (at 25 C)
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2 CI (aq) c. MnO(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl(8) 2+ Mn+ (aq) +...
-
Determine the Norton equivalent at terminals a-b for the circuit in Fig. 4.115 . 10/, ww- 5 A
-
Leslie Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Leslie's...
-
Euclid acquires a 7-year class asset on May 9, 2022, for $153,000 (the only asset acquired during the year). Euclid does not elect immediate expensing under 179. He does not claim any available...
-
Williams & Sons last year reported sales of $10 million, cost of goods sold (COGS) of $8 million, and an inventory turnover ratio of 2. The company is now adopting a new inventory system. If the new...
-
A ceramic manufacturer promised to deliver 25 crates of vases to a Japanese importer under a "CFR" INTERCOM agreement. During transit, however, a large number of vases were broken. The buyer wants to...
-
A company receives $364, of which $23 is for sales tax. The journal entry to record the sale would include a ?
-
Company Following are comparative statements of cash flows, as reported by The Coca Cola Company in its 2014 annual report: Required: a. Briefly review the consolidated statements of cash flows, and...
-
Give the products of the following reaction, where T is tritium: dioldehydrase Ad- CH CH3C-COH CoIII) coenzyme B12
-
With Example 10.9 on page 494 in mind, determine the number of grooves a transmission grating must have if it is to resolve the sodium doublet in the first-order spectrum. Compare the results of both...
-
Sunlight impinges on a transmission grating that is formed with 5000 lines per centimeter. Does the third-order spectrum overlap the second-order spectrum? Take red to be 780 nm and violet to be 390...
-
Suppose that a grating spectrometer while in vacuum on Earth sends 500-nm light off at an angle of 20.0 in the first-order spectrum. By comparison, after landing on the planet Mongo, the same light...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


