Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2
Question:
Use tabulated electrode potentials to calculate ΔG°rxn for each reaction at 25 °C.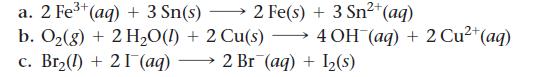
Transcribed Image Text:
2+ a. 2 Fe³+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn²+ (aq) b. O₂(g) + 2 H₂O(l) + 2 Cu(s) →→→ 4 OH(aq) + 2 Cu²+ (aq) c. Br₂(1) + 21 (aq) 2 Br (aq) + 1₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate Grxn for each reaction at 25 C using tabulated electrode potentialswe can use the follo...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2 CI (aq) c. MnO(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl(8) 2+ Mn+ (aq) +...
-
Use tabulated standard electrode potentials to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 C. (The equation is balanced.) Al(s) +...
-
Use the tabulated electrode potentials to calculate G for the reaction Is the reaction spontaneous? I(s) + 2 Br (aq) 21 (aq) + Br(1)
-
Describe at least one method by which the organization can reduce change resistance?
-
Ron LaTulip oversees projects for ACE Construction Company-Recently, the companys controller sent him a performance report regarding the construction of the Campus Highlands Apartment Complex, a...
-
Prepare a pro forma income statement and balance sheet for Webb Enterprises Where revenues are expected to grow by 20% in 2016. Make the following assumptions in making your forecast of the firms...
-
15. Using the information in Table 5, suppose we have a bond that after 2 years pays one barrel of oil plus max(0, S2 20.90), where S2 is the year-2 spot price of oil. If the bond is to sell for...
-
CarMD reports that the cost of repairing a hybrid vehicle is falling even while typical repairs on conventional vehicles are getting more expensive. The most common hybrid repair, replacing the...
-
3233 On June 10. a $5,000 account receivable from a customer was written off using the allowance method On November 1, the account was collected from the customer. How would the reinstatement of the...
-
Calculate the equilibrium constant for each of the reactions in Problem 65. Problem 65 Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2...
-
Which metal is the best reducing agent? a. Mn b. Al c. Ni d. Cr
-
Describe how you would design an orientation program. Be sure to indicate the content of your program and what knowledge and information employees will acquire from attending the program. What are...
-
3. Two companies (A and B) are duopolists that produce identical products. Demand for the products is given by the following demand function: P = 10,000 QA- QB - where QA and QB are the quantities...
-
Consider the following initial-value problem. f'(x) = 2ex - 6x; f(0) = 4 Integrate the function f'(x). (Remember the constant of integration.) || | f'(x)dx = Find the value of C using the condition...
-
The value chain is based on primary activities logstica Operations External logistics Marketing and sales Service and are complemented by support activities Company infrastructure is what it is,...
-
On average, both arms and hands together account for 13% of a person's mass, while the head is 7.0% and the trunk and legs account for 80%. We can model a spinning skater with her arms outstretched...
-
8. Look at the image to the right. Using the Law of Force and Acceleration, predict how acceleration would change if you changed the mass of the boy. 9. Using the same picture from #8, discuss how...
-
Following are a statement of cash flows (indirect method) for Harris, Inc., for the year ended December 31, 2017, and the firm's balance sheet at December 31, 2016: HARRIS, INC. Balance Sheet At...
-
A bar of length = 1 has one fixed and one free end and stiffness function c(x) = 1 - x. Find the displacement when subjected to a unit force. Pay careful attention to the boundary condition at the...
-
What is the total number of lines a grating must have in order just to separate the sodium doublet ( 1 = 5895.9 , 2 = 5890.0 ) in the third order?
-
Imagine an opaque screen containing 30 randomly located circular holes. The light source is such that every aperture is coherently illuminated by its own plane wave. Each wave in turn is completely...
-
Perform the necessary mathematical operations needed to arrive at Eq. (10.76). E = (-1)+1 2Kspd (p + r) 2 sin [wt k(p + ro)] (10.76)
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


