Calculate the equilibrium constant for each of the reactions in Problem 65. Problem 65 Use tabulated electrode
Question:
Calculate the equilibrium constant for each of the reactions in Problem 65.
Problem 65
Use tabulated electrode potentials to calculate ΔG°rxn for each reaction at 25 °C.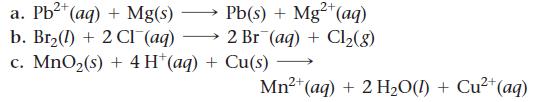
Transcribed Image Text:
a. Pb²+ (aq) + Mg(s) b. Br₂() + 2 CI (aq) c. MnO₂(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl₂(8) 2+ Mn²+ (aq) + 2 H₂O(l) + Cu²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a 531 x ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant for each of the reactions in Problem 66. Problem 66 Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2...
-
Calculate the equilibrium constant for each of the reactions at25 ?C. Standard Electrode Potentials at 25 ?C Reduction Half-Reaction E ?(V) Pb2+( a q )+2 e ? ?Pb( s ) -0.13 Mg2+( a q )+2 e ? ?Mg( s )...
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn+ (aq) b. O(g) + 2 HO(l) + 2 Cu(s) 4 OH(aq) + 2 Cu+ (aq) c. Br(1) + 21 (aq)...
-
1. What are some other ways in which HEALTHeLINK could be used to support public health activities?
-
Prefabricated houses are the specialty of Affordable Homes, Inc., of Corsicana, Texas. Although Affordable Homes produces many models, the companys best-selling model is the Welcome Home, a...
-
Person Electronics manufactures printed circuit boards used in a wide variety of applications ranging from automobiles to washing machines. In fall 2014, it considered whether to invest in two major...
-
14. Using the information in Table 5, suppose we have a bond that pays one barrel of oil in 2 years. a. Suppose the bond pays a fractional barrel of oil as an interest payment after 1 year and after...
-
Sharp Motor Company has two operating divisionsan Auto Division and a Truck Division. The company has a cafeteria that serves the employees of both divisions. The costs of operating the cafeteria are...
-
need belp on both please Question 14 61 point) Which one of the following is not a benefit of budgeting? It requires all levels of management to plan ahead on a recurring basis. It provides definite...
-
Calculate the equilibrium constant for the reaction between Ni 2+ (aq) and Cd(s) (at 25 C)
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2 CI (aq) c. MnO(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl(8) 2+ Mn+ (aq) +...
-
Compare Sections A, B, C, and D in Table 20-2 to determine which type of taxpayer that earns $112,000 would pay the most income tax. Which pays the least?
-
A project requires a $802,000 Initial Investment for equipment. The equipment is estimated to have an eight-year life and a salvage value of $42,000. The project is expected to generate income of...
-
A product has the following costs: $ Per Unit Variable production costs 9.60 Total production costs 15.00 Total variable cost 11.80 Total cost 20.00 22,800 units of the product were manufactured in a...
-
Suppose that Boeing Corporation exported a Boeing 747 to Lufthansa and billed 20 million payable in one year. One-year interest rates are 2% in the United States and 4% in the euro zone. The spot...
-
6. [0/1 Points] DETAILS MY NOTES Find the derivative. f'(x) = f(x) = x9.3x symbolic formatting help
-
1) Explain the following paragraph in your own words. "A nation which has can produce at a lower cost when measured in terms of opportunity cost is said to have a comparative advantage. Even though...
-
Following are comparative balance sheets for Millco, Inc., at January 31 and February 28, 2017: Required: Prepare a statement of cash flows that explains the change that occurred in cash during the...
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
Prove that the equation when applied to a transmission grating, is independent of the refractive index. a(sin 0)m sin ;) ^ [10.61]
-
A grating has a total width of 10.0 cm and contains 600 lines/mm. What is its resolving power in the second-order spectrum? At a mean wavelength of 540 nm, what wavelength difference can it resolve?
-
A high-resolution grating 260 mm wide, with 300 lines per millimeter, at about 75 in autocollimation has a resolving power of just about 10 6 for = 500 nm. Find its free spectral range. How do these...
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...

Study smarter with the SolutionInn App


