Calculate the number of copper atoms in 2.45 mol of copper. SORT You are given the amount
Question:
Calculate the number of copper atoms in 2.45 mol of copper.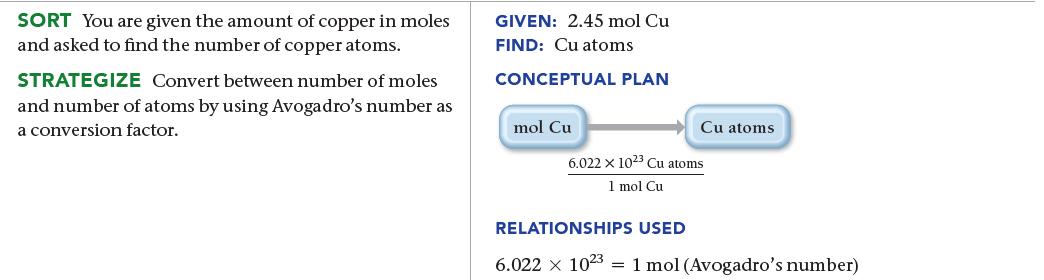
Transcribed Image Text:
SORT You are given the amount of copper in moles and asked to find the number of copper atoms. STRATEGIZE Convert between number of moles and number of atoms by using Avogadro's number as a conversion factor. GIVEN: 2.45 mol Cu FIND: Cu atoms CONCEPTUAL PLAN mol Cu Cu atoms 6.022 x 1023 Cu atoms 1 mol Cu RELATIONSHIPS USED 6.022 x 1023 1 mol (Avogadro's number)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
245 mol Cu x ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many copper atoms are in a copper penny with a mass of 3.10 g? (Assume that the penny is composed of pure copper.) SORT You are given the mass of copper and asked to find the number of copper...
-
In the experiment: Determining Avogadro's Number the values that I've got are Trial 1 Trial 2 Trial 2 Initial mass of Copper electrode (g) 12.517 12.480 12.434 Final mass of Copper electrode (g)...
-
(a) Suppose you have a cube of copper metal that is 0.236 cm on a side with a mass of 0.1206 g. If you know that each copper atom (radius = 128 pm) has a mass of 1.055 10 22 g (you will learn in...
-
At the beginning of the current season, the ledger of Highland Tennis Shop showed Cash $2,500; Inventory $1,700; and Common Stock $4,200. The following transactions were completed during April. Apr....
-
Are sunk costs ever differential costs?
-
Mary Beth uses a torque feeler that consists of a meter-stick held at the 0-cm end with a weight dangling from various positions along the stick. When the stick is held horizontally, torque is...
-
Distinguish between price discrimination and predatory pricing.
-
Mark's Consulting experienced the following transactions for 2018, its first year of operations, and 2019. Assume that all transactions involve the receipt or payment of cash. Transactions for 2018...
-
Multiple Choice Question 94 Using the following balance sheet and income statement data, what is the debt to assets ratio? Current assets Current liabilities Average assets Total assets $19400 13600...
-
The Beef-Up Ranch feeds cattle for Midwestern farmers and delivers them to processing plants in Topeka, Kansas, and Tulsa, Oklahoma. The ranch must determine the amounts of cattle feed to buy so that...
-
What are the main ideas in Daltons atomic theory? How do they help explain the laws of conservation of mass, of constant composition, and of definite proportions?
-
An argon isotope has a mass number of 40 (A = 40). How many neutrons does it contain? (a) 40 (b) 18 (c) 22
-
Solve the linear Programming problem in problem using the simplex method. Maximize subject to P = 10x1 + 50x2 + 10x3 3x1 + 3x2 + 3x3 66 6x1 - 2x2 + 4x3 48 3x1 + 6x2 + 9x3 108 x1, x2, x3 0
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
The following table contains the monthly operating costs of a company. Salary is not included. Determine the variance and standard deviation of the costs. Enero Febrero Marzo Abril Mayo Junio Julio...
-
Becker & Smith, CPAs, performs a financial statement review for BAM Markets ( BAM ) . Caroline, the manager on the job, learns that Don, a member of the review team, violated the independence rules....
-
Presented here are selected transactions for Sheridan Inc. during August of the current year. Sheridan uses a perpetual inventory system. It estimates a return rate of 10%, based on past experience....
-
. Complete both parts (a) and (b) below. ). In1 (a) Let X11, X12, ..., X be a random sample of size n from a population with mean and variance . Let X21, X22,..., X2n2 be a random sample of size n...
-
In a population, the frequencies of two alleles are B = 0.67 and b = 0.33. The genotype frequencies are BB = 0.50, Bb = 0.37, and bb = 0.13. Do these numbers suggest inbreeding? Explain why or why...
-
The electric field due to a line charge is given by where l is a constant. Show that E is solenoidal. Show that it is also conservative. E =
-
Three of the compounds from Problem 13.14 can be prepared from the reaction between a hydride reducing agent (NaBH 4 or LAH) and a ketone or aldehyde. Identify those three compounds, and explain why...
-
For each of the following alkenes, assign the configuration of the double bond as either E or Z: a. b. c. d. F.
-
Draw the mechanism and predict the product of the following reaction. In this case, H 3 O + must be used as a proton source instead of water. Explain why. 1) xs MeMgBr 2) H*
-
The summarized balance sheets of Cullumber Company and Vaughn Company as of December 31, 2021 are as follows: Cullumber Company Balance Sheet December 31, 2021 Assets $2440000 Liabilities $310000...
-
Rey Company's single product sells at a price of $232 per unit. Data for its single product for its first year of operations follow. $ $ 36 per unit 44 per unit Direct materials Direct labor Overhead...
-
11 15:37 III a Not yet answered Marked out of 2.00 P Flag question Target 5 years total adjusted earnings are: Palla Corporation is considering a mom with fully coming in order to determine offering...

Study smarter with the SolutionInn App


