Classify each organic compound as a hydrocarbon or a functionalized hydrocarbon. For functionalized hydrocarbons, identify the compounds
Question:
Classify each organic compound as a hydrocarbon or a functionalized hydrocarbon. For functionalized hydrocarbons, identify the compound’s family.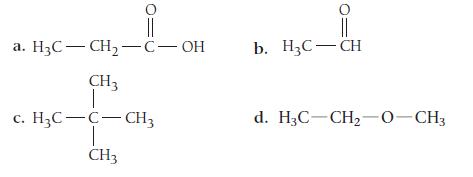
Transcribed Image Text:
a. H3C-CH₂-C-OH CH3 C. H₂C-C-CH3 CH3 || b. H3C-CH d. H3C-CH₂-O-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 16% (6 reviews)
a H3CCH2COH is a functionalized hydrocarbon It belongs to the alcohol family b H3CCH3 is a hydro...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Classify each organic compound as a hydrocarbon or a functionalized hydrocarbon. For functionalized hydrocarbons, identify the compounds family. H3C-CHOH a. b. H3C-CH3 O || C CH - CH3 c. H3C d. HC-NH
-
A 6-arm pulley of dia 650 mm made up of cast iron FG 250, transmits a power of 15kW at 780 rpm. The arms are elliptical cross-sections, in which the major axis is 2.5 times the minor axis. If the...
-
A high-temperature reservoir at T = 500F is to be used as the heat source for a steady-state heat engine, and a low-temperature reservoir at T = 75F is to be used as the heat sink. The boiler takes...
-
Picasso Co. issued 5,000 shares of its $1 par common stock, valued at $100,000, to acquire shares of Seurat Company in an all-stock transaction. Picasso paid the investment bankers $35,000 and will...
-
Consider the following information: (a) Your portfolio is invested 30 percent each in A and C and 40 percent in B. What is the expected return of the portfolio? (b) What is the variance of this...
-
The traction drive uses the capstan drive system shown in Figure CDP2.1. Neglect the effect of the motor inductance and determine a state variable model for the system. The parameters are given in...
-
Explain why an accurate sales forecast is critical to profitability. AppendixLO1
-
1. Why did Nationwide need an enterprise-wide data warehouse? 2. How did integrated data drive the business value? 3. What forms of analytics are employed at Nationwide? 4. With integrated data...
-
Question 42 3 pts Which of the following is a limitation of the accounting rate of return? There is no objective way to choose the cutoff value. O It does not use the relevant information to value a...
-
Imperial Electronics Ltd. is a publicly owned company with 100,000 common shares outstanding. At the last executive committee meeting, Sandra Redgrave, CEO of the company, informed the board members...
-
How many molecules of ethanol (C 2 H 5 OH) (the alcohol in alcoholic beverages) are present in 145 mL of ethanol? The density of ethanol is 0.789 g/cm 3 .
-
Write the formula based on the name, or the name based on the formula, for each hydrocarbon. a. CH 3 CH 3 b. Pentane c. CH 3 CH 2 CH 2 CH 2 CH 2 CH 3 d. Heptane
-
Calculate the aqueous solubility, in moles per liter, of each of the following. (a) BaCrO4, Ksp = 1.2 x 10-10 (b) PbBr2, Ksp = 4.0 10-5 (c) CeF3, Ksp = 8 10-16 (d) Mg3(AsO4)2, Ksp = 2.1 10-20
-
32) Suppose Joaquin grows at an average rate of 0.5in/year for 3 years, then 1.25 inches/year for 4 years, then 0.75 inches/year for 4 years, then 0.4in/year for 5 years. In that time span, how much...
-
Jennifer purchased stock at $50 per share with a 75% initial margin requirement and a maintenance margin of 35%. How much equity per share must Jennifer contribute when the stock falls to $15 per...
-
Thinking about your present job and your "inventory"of leadership traits and characteristics, where are your strengths and weaknesses as a leader?Is being a leader desirable? If yes, what motivates...
-
You are facing a complex decision with several courses of possible action and probabilities associated with them. The current decision tree, based on the best possible estimates of probabilities and...
-
1. In what ways has Marriot proven an industry leader in the context of entrepreneurship in the hospitality industry. 2. What are the author's metrics of measuring entrepreneurial activity, and do...
-
Compare the structural features of a double-stranded RNA structure with those of a DNA double helix.
-
Synthesize the products by drawing out reagents and intermediates along the way. `N H. OH HO HO
-
For a gas at a given temperature, the compression factor z is described by the empirical equation where P° = 1 bar. Calculate the fugacity coefficient for P = 150., 250., 350., 450., and 550....
-
When the following chiral epoxide is treated with aqueous sodium hydroxide, only one product is obtained, and that product is achiral. Draw the product and explain why only one product is formed.
-
When meso-2, 3-epoxybutane is treated with aqueous sodium hydroxide, two products are obtained. Draw both products and describe their relationship.
-
Now the iMost Company is subject to capital rationing and can only afford to adopt one of the projects, the Profitability Index is as follows: Project Y = .73 Project Z = .64 Which project should the...
-
A partial trial balance of Lindy Corporation at December 31, 2020, follows: Dr. Cr. Supplies $7,700 Salaries and wages payable $5,200 Interest receivable 2,690 Prepaid insurance 113,400 Unearned rent...
-
: Star Caf Corporation sells home coffee roasters for an average of OMR 40, and which cost OMR 25. This is a cost-to-retail percentage of 75%. Star Cafes beginning inventory has a cost of OMR 50,000,...

Study smarter with the SolutionInn App


