Complete and balance each combustion reaction equation. a. S(s) + O(g) C. Ca(s) + O(g) b. C3H6(g)
Question:
Complete and balance each combustion reaction equation.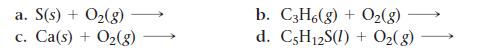
Transcribed Image Text:
a. S(s) + O₂(g) C. Ca(s) + O₂(g) b. C3H6(g) + O₂(g) d. C5H12S(I) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Ss O8 b 2 C3H6g 9 ...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each combustion equation. a. C4H9OH + O2 ( ? b. CH3NO2 + O2 ( ?
-
Complete and balance each combustion equation. a. B2H6 + O2 ( ? (The oxide of boron formed is B2O3.) b. Al2S3 + O2 ( ? (The oxide of sulfur formed is SO2.) c. Al2S3 + O2 ( ? (The oxide of sulfur...
-
Complete and balance each combustion reaction equation: a. C4H6(g) + O(g) C. CS(s) + O(g) b. C(s) + O(g) d. C3H8O(l) + O(g)
-
The Carter Caterer Company must have the following number of clean napkins available at the beginning of each of the next four days: day 1, 1500; day 2, 1200; day 3, 1800; day 4, 600. After being...
-
Consider the following abbreviated financial statements for Cabo Wabo, Inc.: a. What is owners' equity for 2007 and 2008? b. What is the change in net working capital for 2008? c. In 2008, Cabo Wabo...
-
A piston-cylinder device initially contains 0.2 kg of steam at 200 kPa and 300oC. Now, the steam is cooled at constant pressure until it is at 150oC. Determine the volume change of the cylinder...
-
What does it mean for a market to be efficient? AppendixLO1
-
The following selected transactions relate to investment activities of Ornamental Insulation Corporation. The company buys securities, not intending to profit from short-term differences in price and...
-
$7,000 Julia pald the following taxes this year. Calculate the amount of the state and local tax deduction which Julia may include in itemized deductions for 2020. State income tax withholding State...
-
Write a letter to the Smith's discussing the results of their tax return, remind them of any deduction substantiation rules they need to follow (receipts, mileage log, etc.), offer suggestions for...
-
Write a balanced chemical equation for the reaction of solid strontium with iodine gas.
-
What volume of a 1.50 M HCl solution should you use to prepare 2.00 L of a 0.100 M HCl solution? a) 0.300 L b) 0.133 L c) 30.0 L d) 2.00 L
-
Solve the problem. Evaluate each sum that converges. Identify any that diverge. 30 (a) (-3i + 6) (b) (c) i=1 i=1 i=1
-
Y = AK[1-a R P = QAKa-1[1-a W P = (1 -Q) AKL-a 1= 14 1 -4 Y = C
-
Inferring Transactions from Financial Statements (FSET) Wired.com Inc. is a large e-commerce company, with over $31 billion in revenues for the fiscal year ended December 31, 20X2. For the year ended...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
For the past 30 years, the average satisfaction rating for a sushi restaurant has been 3.9 out of 5. If the rating for a sample of 256 people is 4.1 with a standard deviation of 0.5, the critical...
-
Hash collisions occur when more than one item is mapped to the same element in Hash Table's array. What is one way that a Hash Table can handle collisions?
-
The arctic fox has 50 chromosomes (25 per set), and the common red fox has 38 chromosomes (19 per set). These species can interbreed to produce viable but infertile offspring. How many chromosomes...
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
Which of the following compounds would you expect to be most acidic? Justify your choice.
-
Identify which of the following compounds is expected to be a stronger base. Justify your choice. N. N.
-
Predict the product of the following reaction, and propose a mechanism for its formation. Na, CH,OH NH;
-
delet question
-
Texas Petrochemical reported the following April activity for its VC-30 lubricant, which had a balance of 395 qts.@ $4.30 per quart on April 1. Purchases: Apr. 10 Apr. 14 Apr. 20 595 qts @ $4.40 495...
-
A company manufactures and sells a product for $124 per unit. The company's fixed costs are $72,760, and its variable costs are $94 per unit. The company's break-even point in units is: Multiple...

Study smarter with the SolutionInn App


