Complete and balance each gas-evolution equation. a. HNO3(aq) + NaSO3(aq) b. HCl(aq) + KHCO3(aq) c. HC2HO(aq) +
Question:
Complete and balance each gas-evolution equation.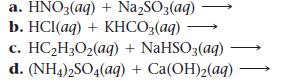
Transcribed Image Text:
a. HNO3(aq) + Na₂SO3(aq) b. HCl(aq) + KHCO3(aq) c. HC₂2H₂O₂(aq) + NaHSO3(aq) d. (NH4)2SO4(aq) + Ca(OH)₂(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The complete and balanced gasevolution equations are as follows a HNO3aq Na2SO3aq H2SO4aq NaNO3aq NO...View the full answer

Answered By

Usman Nasir
I did Master of Commerce in year 2009 and completed ACCA (Association of Chartered Certified Accountants) in year 2013. I have 10 years of practical experience inclusive of teaching and industry. Currently i am working in a multinational company as finance manager and serving as part time teacher in a university. I have been doing tutoring via many sites. I am very strong at solving numerical / theoretical scenario-based questions.
4.60+
16+ Reviews
28+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each combustion equation. a. C4H9OH + O2 ( ? b. CH3NO2 + O2 ( ?
-
Complete and balance each combustion equation. a. B2H6 + O2 ( ? (The oxide of boron formed is B2O3.) b. Al2S3 + O2 ( ? (The oxide of sulfur formed is SO2.) c. Al2S3 + O2 ( ? (The oxide of sulfur...
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
why is teamwork so important especially in healthcare? explain
-
Why is it important for managers to evaluate internal controls?
-
From the following trial balance for E Soormally, produce a statement of comprehensive income for the period ending 30 September 2017 and a statement of financial position as at that date. Inventory...
-
What, if any, planning assistance might Stacey request from her regional human resources department since the program is managed by a contract management company?
-
Higgs Bassoon Corporation is a custom manufacturer of bassoons and other wind instruments. Its current value of operations, which is also its value of debt plus equity, is estimated to be $200...
-
6. Refer to the information below to see how Kaminsky Insurance Agency reported its expenses as of June 30th. Prepare T-accounts and insert the account balances prior to closing. Post the closing...
-
ABC Company - Biographical Information ABC Company is a family owned business which Jonathan started 15 years ago, issuing 900 shares of the 1000 authorized, at a par value of $100 a share. ABC...
-
Assign oxidation states to each atom in each element, ion, or compound. a. Ag d. HS b. Ag e. CO3- c. CaF f. CrO4- 2-
-
Complete and balance each gas-evolution equation. a. HBr(aq) + NIS(s) b. NH4l (aq) + NaOH(aq) c. HBr(aq) + NaS(aq) d. HCIO4(aq) + LiCO3(aq)
-
If Nicaragua can produce with the same amount of resources twice as much coffee as Colombia, explain how Colombia could have a comparative advantage in producing coffee?
-
15.5 please help will give like if answers r correct Exercise 15-8 (Static) Sales-type lease with selling profit; lessor; calculate lease payments [LO15-3] Manufacturers Southern leased high-tech...
-
When my son was young, he had 8 different plastic dinosaurs to arrange. How many ways could he arrange his 8 dinos? He had favorite dinos, so placing them in proper order was very important. How many...
-
Process P1 init (mutEx); num = 0; loop1 = 0; while (loop1 < 3) wait (mutEx); num num + 1; signal (mutEX); loop1 loop1 + 1; Process P2 loop2 = 0; while (loop2 < 2) wait (mutEx); num num + 10;...
-
PROBLEM 3-5B Following is the chart of accounts of Smith Financial Services: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Office Furniture Liabilities 221 Accounts...
-
4. Identify a service you could refer Casey to and write a referral for her (up to 300 words).
-
Determine the relative amounts (in terms of mass fractions) of the phases for the alloys and temperatures given in Problem 9.8. Problem 9.8. (a) 90 wt% Zn-10 wt% Cu at 400C (750F) (b) 75 wt% Sn-25...
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
An oil is tested using a Saybolt viscometer and its viscosity is 526 SUS at 40C. Determine the kinematic viscosity of the oil in mm 2 /s at that temperature.
-
Convert all of the kinematic viscosity data in Table 2.5 for ISO viscosity grades from mm 2 /s (cSt) to SUS. Kinematic Viscosity at 40C (cSt) or (mm2/s) Grade IS VG Nominal Minimum Maximum 2.2 1.98...
-
Convert 1600 square millimeters to square meters.
-
A total of 2,000 units of Product A are produced from a joint process. Product A can be sold at the split-off point for $16 per unit, or it can be processed further for an additional total cost of...
-
How has COVID - 19 affected the job market in Canada Summary of all research conducted with a minimum of 3 credible sources? An activity you will be using to engage the audience and enforce the...
-
please as soon as can i will rate the thumps up IF THE BANK GIVES YOU 5% INTEREST ON YOUR SAVING ACCOUNT AND INFLATION IS 3% WHAT IS YOU Select one: O a. CANNOT BE CALCULATED b. 1.94% O c. 2% O d....

Study smarter with the SolutionInn App


