Consider the generic chemical equation A + 3B 2C. Let circles represent molecules of A and
Question:
Consider the generic chemical equation A + 3B → 2C.
Let circles represent molecules of A and squares represent molecules of B. The diagram shown at the far right represents the amount of B available for reaction. Which diagram in the answer options accurately represents the amount of A necessary to completely react with B?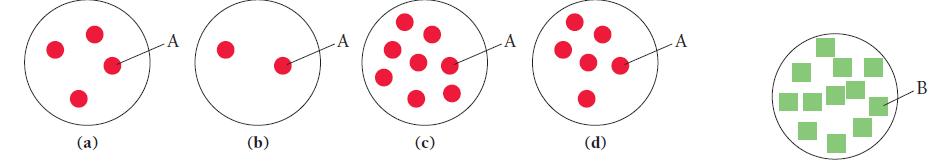
Transcribed Image Text:
(a) A (b) -A (c) A (d) A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Since the balanced equation indic...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Under certain conditions, sodium reacts with oxygen to form sodium oxide according to the reaction: A flask contains the amount of oxygen represented by the diagram shown at far right. Which of the...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Assume the premises: S. S-P. P~N, ~R-N. Give a detailed informal proof of: R. Number each step and give an explanation for each. Write all the steps in the proof at once in the Math Editor. (it works...
-
Data for Ulrich Company are presented in P12-9B. Further analysis reveals that accounts payable pertain to merchandise creditors. Instructions Prepare a statement of cash flows for Ulrich Company...
-
Consider a building whose annual air-conditioning load is estimated to be 40,000 kWh in an area where the unit cost of electricity is $0.10/kWh. Two air conditioners are considered for the building....
-
Explain why analysts added back depreciation expense to compute operating cash flow in Exhibit 14-9.
-
Northwest Desserts, Inc., (NDI) produces a variety of premium cheesecakes and sells them in individual packages directly to retail customers and in packages of 10 cakes to restaurants in Washington,...
-
Required information Use the following information for Exercises 2 5 - 2 7 below. ( Algo ) [ The following information applies to the questions displayed below. ] - Carmen Camry operates a consulting...
-
To keep track of office furniture, computers, printers, and so on, the FOUNDIT Company uses the table structure shown in Table P6.5. a. Given that information, write the relational schema and draw...
-
Sodium and chlorine react to form sodium chloride: What is the theoretical yield of sodium chloride for the reaction of 55.0 g Na with 67.2 g Cl 2 ? a) 1.40 * 10 2 g NaCl b) 111 g NaCl c) 55.4 g NaCl...
-
Identify the reactants and products in this chemical equation. 4 NH 3 (g) + 5 O2( g) 4 NO(g) + 6 H 2 O(g)
-
Oaks Company had the following balances in its accounting records as of December 31, 2010. The following accounting events apply to Oaks's 2010 fiscal year: Jan. 1 Acquired an additional $70,000 cash...
-
Using what you know of groups and teams, how do you get your teams back on track with Pat? What actions do you need to take as a leader to minimize/repair the negative impact Pat's has had on the...
-
The demand function for a certain product is given by p = 3000 2x + 100 (0 x 10) where x (measured in units of a thousand) is the quantity demanded per week and p is the unit price in dollars. Sketch...
-
QUESTION 4 Dr. Martin Luther King's Speech where he proclaimed, "Free at last! Free at last!" is an example of O A. Impromptu O B. Dissolving OC. Prepared D. Reference E.Planned Speaking Wording...
-
If the majority voting control partners in an entity are close to retirement, they may prefer more equity issued versus debt. T/F The more stable the selling price is, the more likely the firm ...
-
Explain the following concepts/topics. 1. Arbitration Tribunials under CUSMA as opposed to International Arbitration under the WTO or ICSID: 2. Lost or Not Lost clauses under a Marine Insurance...
-
Pick any example of a genetic technology and describe how it has directly affected your life.
-
On March 31, 2018, Gardner Corporation received authorization to issue $30,000 of 9 percent, 30-year bonds payable. The bonds pay interest on March 31 and September 30. The entire issue was dated...
-
The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72 kJ mol -1 at 1 atm pressure. Calculate the normal and standard boiling points. Does your result for...
-
Benzene(l) has a vapor pressure of 0.1269 bar at 298.15 K and an enthalpy of vaporization of 30.72 kJmol 1 . The C P,m of the vapor and liquid phases at that temperature are 82.4 and 136.0 J K 1 mol...
-
Use the values for G o f (CCl 4 , l) and G o f (CCl 4 , g) from Appendix B to calculate the vapor pressure of CCl 4 at 298.15 K.
-
Digi Control International Inc. manufactures robotic controllers in Division A, a country with a 30% income tax rate, and transfers them to Division B, a country with a 40% income tax. An import duty...
-
The cost of an asset as well as its accumulated depreciation is removed when an asset is sold. True or False
-
In which of the following scenarios will a taxpayer NOT be eligibile to make a voluntary contribution on their Iowa return. Linda is due Iowa refund of $213 and wishes to donate $100 to the Am Heart...

Study smarter with the SolutionInn App


