Consider the reaction in which HCl adds across the double bond of ethene: HCl + H 2
Question:
Consider the reaction in which HCl adds across the double bond of ethene:
HCl + H2C = CH2→ H3C—CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction: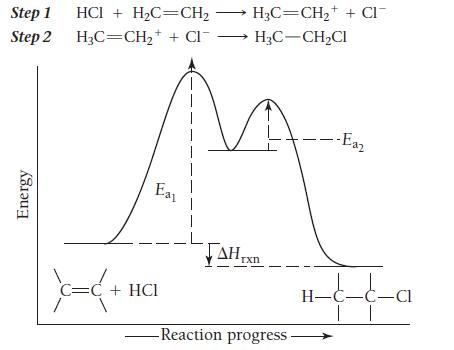
a. Based on the energy diagram, determine which step is rate limiting.
b. What is the expected order of the reaction based on the proposed mechanism?
c. Is the overall reaction exothermic or endothermic?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





