Determine the coordination number for each structure. (a) Gold (b) Ruthenium
Question:
Determine the coordination number for each structure.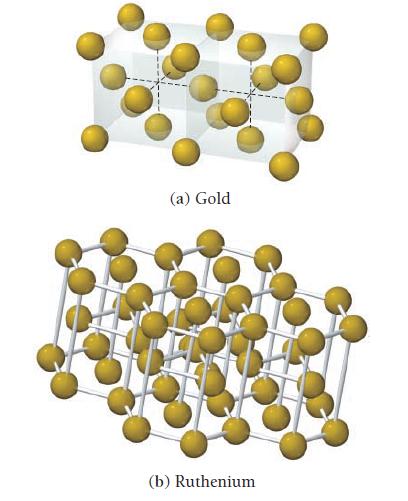
Transcribed Image Text:
(a) Gold (b) Ruthenium
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The coordination number for each structure in the image is as fo...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sodium oxide (Na 2 O) adopts a cubic structure with Na atoms represented by green spheres and O atoms by red spheres. (a) How many atoms of each type are there in the unit cell? (b) Determine the...
-
The coordination number for the AI3+ ion is typically between four and six. Use the anion coordination number to determine the coordination number in the following compounds: (a) AlF3 where the...
-
The coordination number for Mg2+ ion is usually six. Assuming this assumption holds, determine the anion coordination number in the following compounds: (a) MgS, (b) MgF2, (c) MgO.
-
Select the reasons why most professional telescopes are reflectors rather than refractors. A mirror can collect light more efficiently than a lens. Reflecting telescopes have shorter focal lengths...
-
Serges is the owner of a retail meat marketing business. Without authority his managing agent borrowed $3,500 from David on Sergess behalf, for use in Sergess business. Serges paid $200 on the...
-
FDR Partners is a large regional partnership. This year, Rusty acquired an interest in the firm by purchasing it from an existing partner. The firms balance sheet just prior to Rustys acquisition was...
-
Compute and interpret the price- earnings ratio. AppendixLO1
-
Larry has been the chief financial officer (CFO) of Maxima Auto Service for the past 10 years. The company has reported profits each year it's been in business. However, this year has been a tough...
-
Check my work Alyeski Tours operates day tours of coastal glaciers in Alaska on its tour boat the Blue Glacier. Management has identified two cost drivers--the number of cruises and the number of...
-
Change Purse Inc. is a small business that is planned to be located in a small Nova Scotia town. The town was incorporated in 1889 and, like many communities in Nova Scotia, it prides itself on being...
-
Calculate the packing efficiency of the body-centered cubic unit cell. Show your work.
-
An X-ray beam of unknown wavelength is diffracted from a NaCl surface. If the interplanar distance in the crystal is 286 pm, and the angle of maximum reflection is found to be 7.23, what is the...
-
Examine two national labor unions, such as the UAW and SEIU. What are the issues these unions are concerned with? a. Are the issues the same for both unions? b. Why do you think these issues are...
-
The Capital Asset Pricing Model (CAPM) says that the risk premium on security \(j\) is related to the risk premium on the market portfolio, that is where \(r_{j}\) and \(r_{f}\) are the returns to...
-
Verify the likelihood (8.16). n t-1 II (Pt, (xi) i (1 Pt, (x;))(1-cs) II (1 Pk (xi)) - (8.16) i=1 k=1
-
Check that [edgm \(][\) cdgm \(]\) is a graphical model. Education Gender MS Dep CIRS
-
Use the information provided in P3-9B. Required a. Prepare closing entries at December 31 in general journal form using the Income Summary account. b. After the closing entries are posted, calculate...
-
If the light bulb in Figure \(33.8 a\) is \(1.0 \mathrm{~m}\) in front of the mirror, how far behind the mirror is the image? Data from Figure 33.8a (a) Rays shows path of light that travels from...
-
Draw the two stereoisomers of 1,3-dimethylcyclobutane, and classify the pair according to the categories listed in A, B, and C above.
-
Would you use the adjacency matrix structure or the adjacency list structure in each of the following cases? Justify your choice. a. The graph has 10,000 vertices and 20,000 edges, and it is...
-
How long will it take to pass 200. mL of H 2 at 273 K through a 10.0-cm-long capillary tube of 0.250 mm if the gas input and output pressures are 1.05 and 1.00 atm, respectively?
-
a. Derive the general relationship between the diffusion coefficient and viscosity for a gas. b. Given that the viscosity of Ar is 223 P at 293K and 1.00 atm, what is the diffusion coefficient?
-
a. Derive the general relationship between the thermal conductivity and viscosity. b. Given that the viscosity of Ar is 223 P at 293K and 1atm, what is the thermal conductivity? c. What is the...
-
1) A portfolio consists for four securities A, B, C &D have the following expected rate of return and portfolio value invested. Security Expected Return Proportion of investment A 15% 30% B 12% 20% C...
-
A firm financed by equity and debt only has financial risk. (Your answer must begin with True or False followed by your explanation.) The level of debt in a firm does not affect the firms valuation...
-
Level 2 - Managing Purchases for Brightstar Toy Company Brightstar Toy Company, a national toy store, is planning a huge promotion for Power Blocks action figures during the upcoming holiday season....

Study smarter with the SolutionInn App


